What Is Chromatography?
Chromatography is a cornerstone technique in biochemical analysis and research, widely used to separate, identify, and quantify complex mixtures. The method relies on differential interactions between substances and a stationary phase, allowing precise analysis of a wide range of compounds.
Chromatography is a separation technique used to isolate individual components from a mixture based on their different affinities for two phases: the stationary phase and the mobile phase. The term "chromatography" is derived from the Greek words "chroma" (color) and "grapho" (to write), reflecting its origins in the separation of colored compounds. Today, chromatography is used in fields ranging from environmental analysis to pharmaceutical development.
Principles of Chromatography
Stationary Phase: This phase remains fixed in place and can be solid or liquid. The stationary phase interacts with the sample components differently, leading to their separation. Common stationary phases include silica gel, alumina, and various polymeric materials.
Mobile Phase: This phase moves through or over the stationary phase and can be a liquid or gas. The choice of mobile phase affects the separation efficiency and is tailored to the nature of the sample and stationary phase.
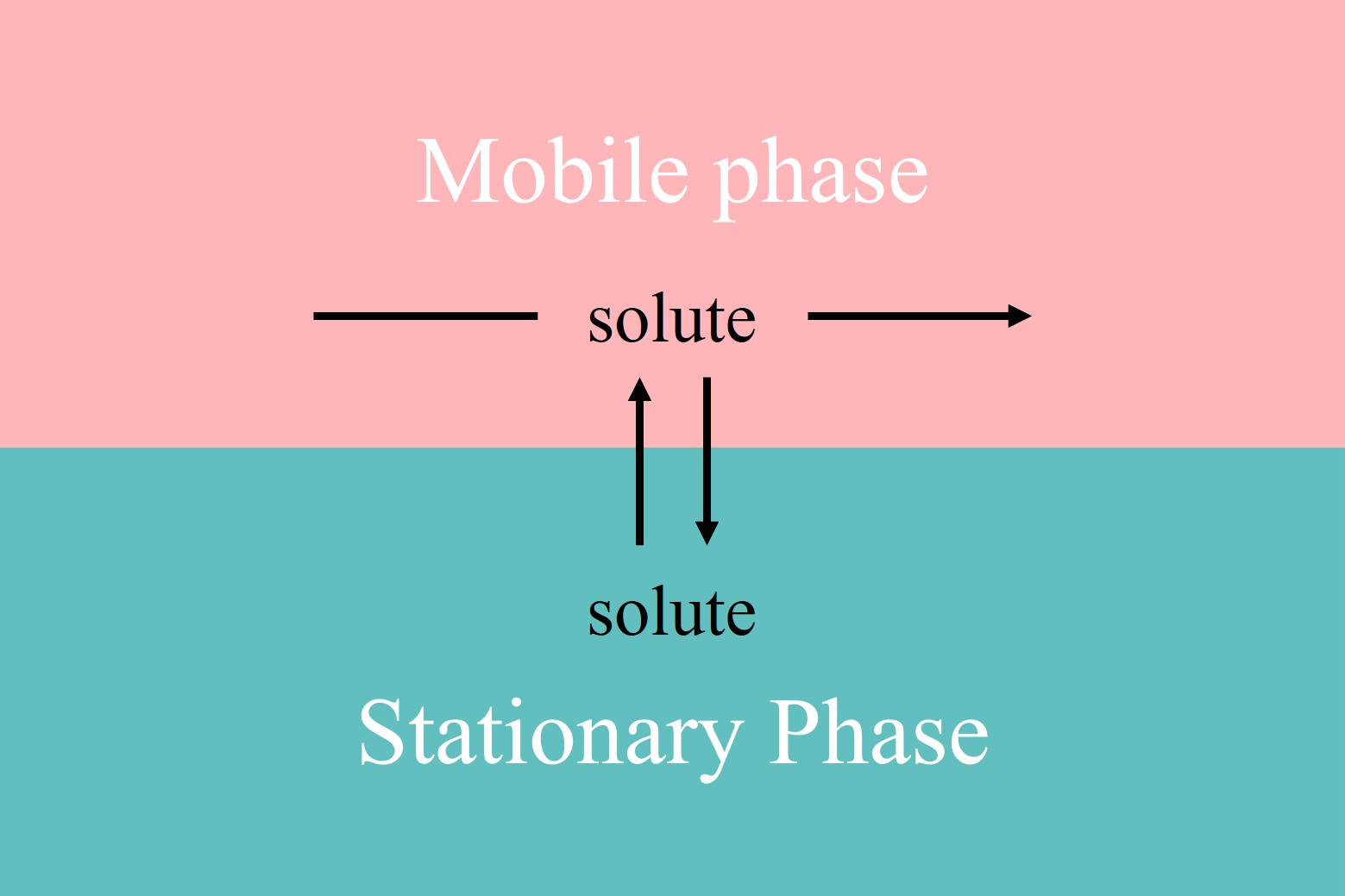 Figure 1. The mobile phase containing the solute moves through or over the stationary phase. When a sample is injected into a mobile phase, the components of the sample both move with the mobile phase and partition into the stationary phase.
Figure 1. The mobile phase containing the solute moves through or over the stationary phase. When a sample is injected into a mobile phase, the components of the sample both move with the mobile phase and partition into the stationary phase.
Retention and Elution: As the mobile phase carries the sample through the stationary phase, different components interact with the stationary phase to varying degrees. Components that interact more strongly with the stationary phase move more slowly, while those that interact less move more quickly. This differential movement results in the separation of the components as they elute from the stationary phase.
Types of Mobile Phases and Stationary Phases
In chromatography, the stationary phase and the mobile phase are fundamental components that define the separation mechanism of different methods. While the principle behind these phases remains consistent - separation based on differential affinities between the analyte and the two phases - the nature of these phases varies between different chromatographic techniques.
The following table summarizes the stationary and mobile phases used in different chromatography techniques.
Table 1. Stationary phase and mobile phase used in different chromatographic methods.
| Chromatographic Method | Stationary Phase | Mobile Phase |
| Gas Chromatography (GC) | Liquid or solid coating | Inert gas (e.g., helium) |
| Liquid Chromatography (LC) | Solid adsorbent (silica, modified silica) | Liquid solvent (e.g., water, methanol) |
| Thin-Layer Chromatography (TLC) | Solid adsorbent (e.g., silica gel) | Liquid solvent (e.g., ethyl acetate, hexane) |
| Ion-Exchange Chromatography (IEC) | Ion-exchange resin | Aqueous buffer (e.g., NaCl) |
| Size-Exclusion Chromatography (SEC) | Porous gel or polymer beads | Aqueous buffer or organic solvent |
| Affinity Chromatography | Ligand-immobilized beads | Buffer solutions |
| Paper Chromatography | Water adsorbed in cellulose fibers | Liquid solvent |
General Workflow of Chromatography
Sample Introduction: The sample is introduced into the chromatographic system. In liquid chromatography (LC), the sample is injected into the mobile phase. In gas chromatography (GC), the sample is vaporized and carried by the gas mobile phase.
Separation: The sample mixture moves through the stationary phase where the components interact differently with the stationary phase. This interaction is influenced by several factors including polarity, size, charge and affinity.
Detection: As components elute from the stationary phase, they are detected by a suitable detector. The detector provides data on the identity and concentration of each component, typically resulting in a chromatogram—a graphical representation of the separation process.
Interaction Mechanisms of Chromatography
Adsorption: Components adhere to the stationary phase based on their surface interactions. This mechanism is used in adsorption chromatography and is influenced by the chemical properties of both the stationary phase and the sample.
Partitioning: Components distribute between two immiscible phases, typically a liquid stationary phase and a liquid mobile phase. This principle is used in partition chromatography, such as reversed-phase chromatography (RPC).
Size Exclusion: Separation is based on the size of the molecules, with larger molecules eluting faster because they cannot enter the pores of the stationary phase. This mechanism is used in size exclusion chromatography (SEC).
Ion Exchange: Components are separated based on their charge interactions with the stationary phase. This mechanism is used in ion exchange chromatography (IEC), in which components with opposite charges are attracted to the stationary phase.
Classification of Chromatography
In general, based on the physical state of the mobile phase, chromatography can be divided into gas chromatography and liquid chromatography. Gas chromatography can be divided into two types according to the different stationary phases: gas-solid chromatography, in which the solid surface is a stationary phase that retains the analytes on it by the process of physical adsorption, and gas-liquid chromatography, in which the liquid stopped on a solid is the stationary surface. This type of chromatography almost always involves the use of a packed column. Liquid chromatography involves the use of a mobile phase that exists in a liquid state. Liquid chromatography can be performed either on a plate or in a column. It should be noted that there are many subcategories of liquid chromatography such as ion exchange chromatography (IEC) and size-exclusion chromatography (SEC).
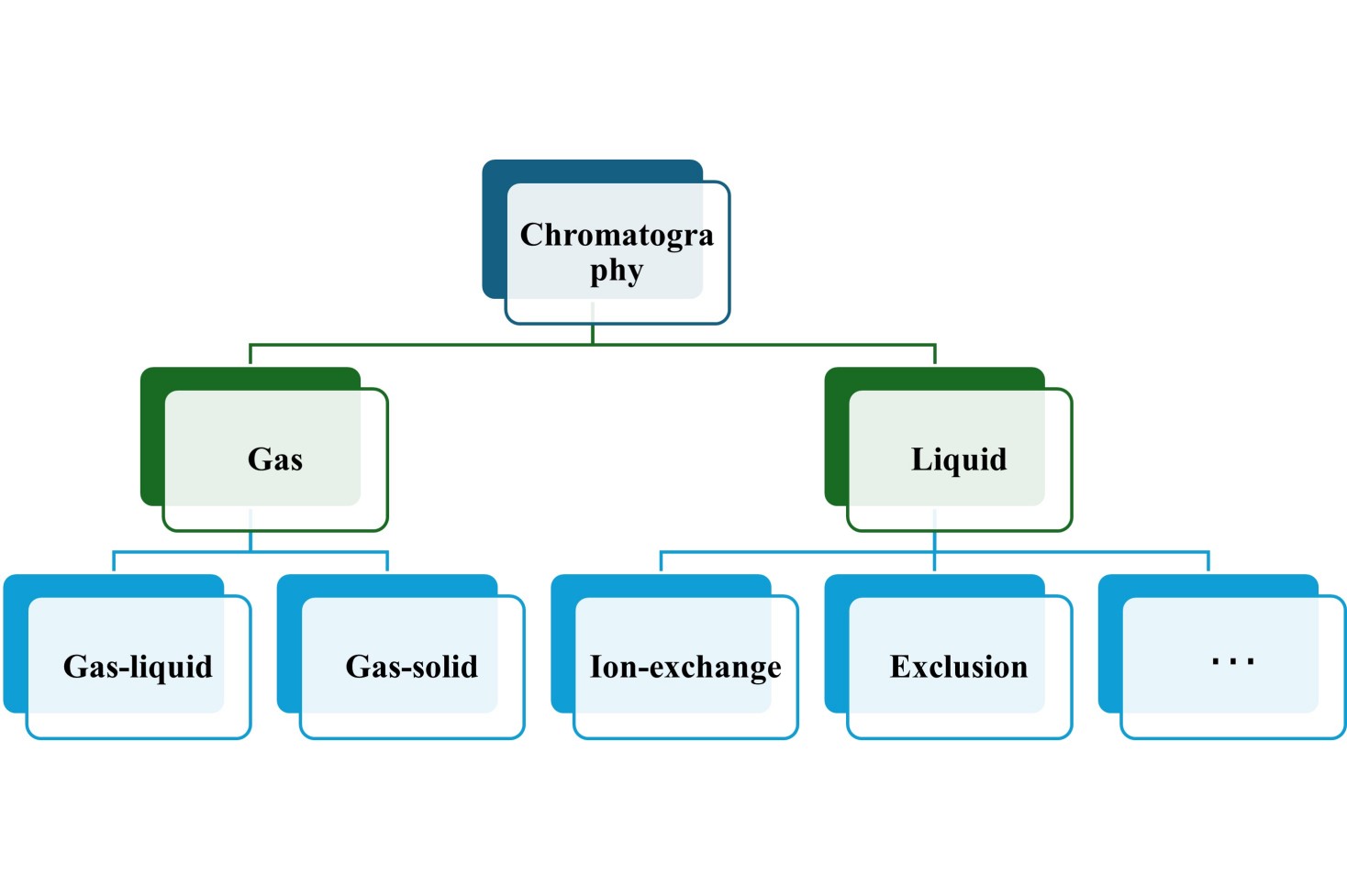 Figure 2. Classification of chromatography based on the physical state of the mobile phase.
Figure 2. Classification of chromatography based on the physical state of the mobile phase.
The main types of chromatography used in structural biology are summarized in the table below:
Table 2. Main types of chromatography and their separation principles and applications.
| Gel Filtration Chromatography (Size-Exclusion Chromatography) | This method separates molecules based on their size. Larger molecules elute first as they bypass small pores in the stationary phase, making this technique ideal for studying protein complexes and determining molecular weight. |
| Ion-Exchange Chromatography | This technique separates biomolecules based on their charge by exploiting the interactions between charged molecules and the oppositely charged groups on the stationary phase. It is often used to purify proteins or nucleic acids, study protein interactions, and isolate specific subtypes of molecules. |
| Affinity Chromatography | This technique separates molecules based on specific binding interactions between a target molecule and a ligand attached to the stationary phase. It is commonly used for purifying proteins with high specificity, such as those with tagged fusion proteins or antibodies. |
| Hydrophobic Interaction Chromatography | It separates molecules based on their hydrophobicity by exploiting the interaction of hydrophobic regions on the biomolecule with the stationary phase. This is particularly useful for studying proteins in their native state. |
| Reversed-Phase Chromatography | This technique involves a hydrophobic stationary phase and separates molecules based on their hydrophobicity. It is often used for analyzing peptides and small proteins after enzymatic digestion. |
Applications of Chromatography
Chromatography techniques are indispensable in structural biology, where they are used to purify, analyze, and characterize biomolecules. These applications are critical for understanding molecular structures and functions.
Protein Purification: Chromatography techniques such as fast protein liquid chromatography (FPLC), SEC, and affinity chromatography are fundamental for purifying proteins from complex biological mixtures. These methods allow researchers to isolate specific proteins in their native state for further study. For example, protein A affinity chromatography is widely used to purify antibodies by leveraging their specific interactions with Protein A. This technique is crucial for obtaining high-purity antibodies for structural and functional analyses.
Determination of Molecular Weight and Size: SEC and HDC are employed to determine the molecular weight and size of proteins, nucleic acids, and other biomolecules. Accurate size determination is essential for understanding the oligomeric state and structural properties of these molecules. SEC coupled with multiangle light scattering (MALS) provides precise measurements of protein size and molar mass distribution, which are critical for characterizing protein complexes and aggregates.
Characterization of Protein-Ligand Interactions: Chromatography techniques, including affinity chromatography and two-dimensional chromatography (TDC), are used to study interactions between proteins and their ligands. These studies provide insights into binding affinities, stoichiometry, and the nature of the interactions. Surface plasmon resonance (SPR) combined with chromatography can analyze protein-ligand binding kinetics, providing detailed information about interaction strengths and binding dynamics.
Study of Post-Translational Modifications: Chromatography is employed to analyze post-translational modifications of proteins, such as phosphorylation, glycosylation, and acetylation. These modifications play a significant role in protein function and regulation. Affinity chromatography with specific antibodies can enrich phosphorylated proteins for mass spectrometry analysis, facilitating the study of phosphorylation patterns and their functional implications.
Separation and Analysis of Nucleic Acids: Chromatography techniques such as ion exchange chromatography and reversed-phase chromatography are used to separate and analyze nucleic acids, including DNA and RNA. These methods are crucial for studying gene expression, sequencing, and other genetic analyses. Ion exchange chromatography is used to purify plasmid DNA for sequencing and cloning applications, ensuring high-quality samples for downstream analyses.
As chromatography techniques continue to evolve, their applications will expand, offering deeper insights into molecular structures and functions. The advancements in chromatography, such as nanoparticle-based methods, microfluidic devices, and integrated systems, promise to further enhance the capabilities of this powerful analytical tool.
At Creative Biostructure, we specialize in providing a comprehensive range of chromatographic analyses tailored for structural biology research. Our cutting-edge technologies ensure precise separation and identification of biomolecules. Whether you're analyzing proteins, nucleic acids, or small molecules, our expert team is ready to support your scientific discoveries. Contact us today to discuss how our chromatographic solutions can accelerate your research and optimize your results.
References
- Lehninger Principles of Biochemistry, Fifth Edition, 2008. W.H. Freeman and Company.
- Encyclopedia of analytical science, Elsevier, 2010. Worsfold, P. J., Townshend, A., & Poole, C. F.
- Brewer, A. K. (2021). Hydrodynamic chromatography: The underutilized size-based separation technique. Chromatographia, 84(9), 807–811.
- Coskun, O. (2016). Separation techniques: Chromatography. Northern Clinics of Istanbul, 3(2), 156–160.
- Isenberg, S. L., Brewer, A. K., Côté, G. L., & Striegel, A. M. (2010). Hydrodynamic versus size exclusion chromatography characterization of alternan and comparison to off-line MALS. Biomacromolecules, 11(9), 2505–2511.
- Marston, A. (2007). Role of advances in chromatographic techniques in phytochemistry. Phytochemistry, 68(22–24), 2786–2798.
- Thikekar, A. K., Rathod, V. S., Panchal, V. P., Raut, S. A., Raut, R. S., & Jain, K. S. (2023). A review on-analytical tools in proteomics. Journal of Proteins and Proteomics, 14(3), 201–221.