Cryo-Electron Tomography (Cryo-ET) Technology
Cryo-electron tomography (cryo-ET) is a cutting-edge technique in structural biology that allows for the 3D visualization of biological specimens at nanometer-scale resolution. Unlike single-particle Cryo-Electron Microscopy (Cryo-EM), which averages multiple images to create a structure, cryo-ET captures individual snapshots of samples, enabling the study of heterogeneous or dynamic systems. This makes cryo-ET invaluable for analyzing the architecture of cells and their macromolecular complexes in situ, providing a unique insight into biological structures in their native environments.
Principles and Instrumentation of Cryo-ET
Cryo-ET is based on the principles of electron microscopy and tomography. The basic principle is to take 2D images of a frozen sample from multiple angles and then computationally reconstruct them into a 3D volume. This tomographic reconstruction provides a detailed view of the internal organization of cells, tissues, or macromolecular complexes.
The instrumentation for cryo-ET is similar to cryo-EM but includes additional features for tilting the sample and collecting data from different angles. The core components are:
Electron Source: A high-energy electron beam is generated, typically using a field emission gun (FEG), to provide the resolution required to capture fine structural details.
Cryo-Sample Holder: The biological sample, vitrified in vitreous ice, is placed in a holder that can be tilted at various angles to collect data from different orientations.
Goniometer: This device allows for precise rotation of the sample to capture images at a range of tilt angles, usually between -70° and +70°.
Electron Optics and Detectors: Electromagnetic lenses focus the electron beam, and the scattered electrons are recorded by highly sensitive detectors, typically direct electron detectors, which offer fast, high-resolution imaging.
Workflow of Cryo-ET
The cryo-ET process is divided into the following steps:
Vitrification: The sample type usually dictates the vitrification method. Samples that are thin enough to be directly investigated by EM are commonly vitrified by plunge-freezing into a cryogen where sufficiently fast cooling speeds are reached. High-pressure freezing combines rapid temperature decrease with the application of high pressure, which lowers the melting point of water. This allows vitrification of samples up to 200 µm in thickness, but cryo-protectants are often needed.
Thinning: Due to a strong electron–matter interaction, samples thicker than 1 µm are virtually opaque to electrons and require thinning at cryo-conditions. Although vitreous sectioning is currently used more often, focused ion beam milling (FIB) is an emerging method with a great promise for the future.
Electron tomography: The goniometer tilts the sample to capture images from multiple angles, typically between -70° and +70°. These 2D images, called tilt series, are then computationally reconstructed into a 3D volume using tomographic reconstruction algorithms. The resulting 3D tomograms provide detailed information about the internal structure of the sample.
Data analysis: Computational methods are used to improve the resolution of structures visualized in tomograms and to aid in their molecular identification. Cryo-correlative light microscopy is an optional procedure that facilitates identification and navigation to target features in cryo-ET.
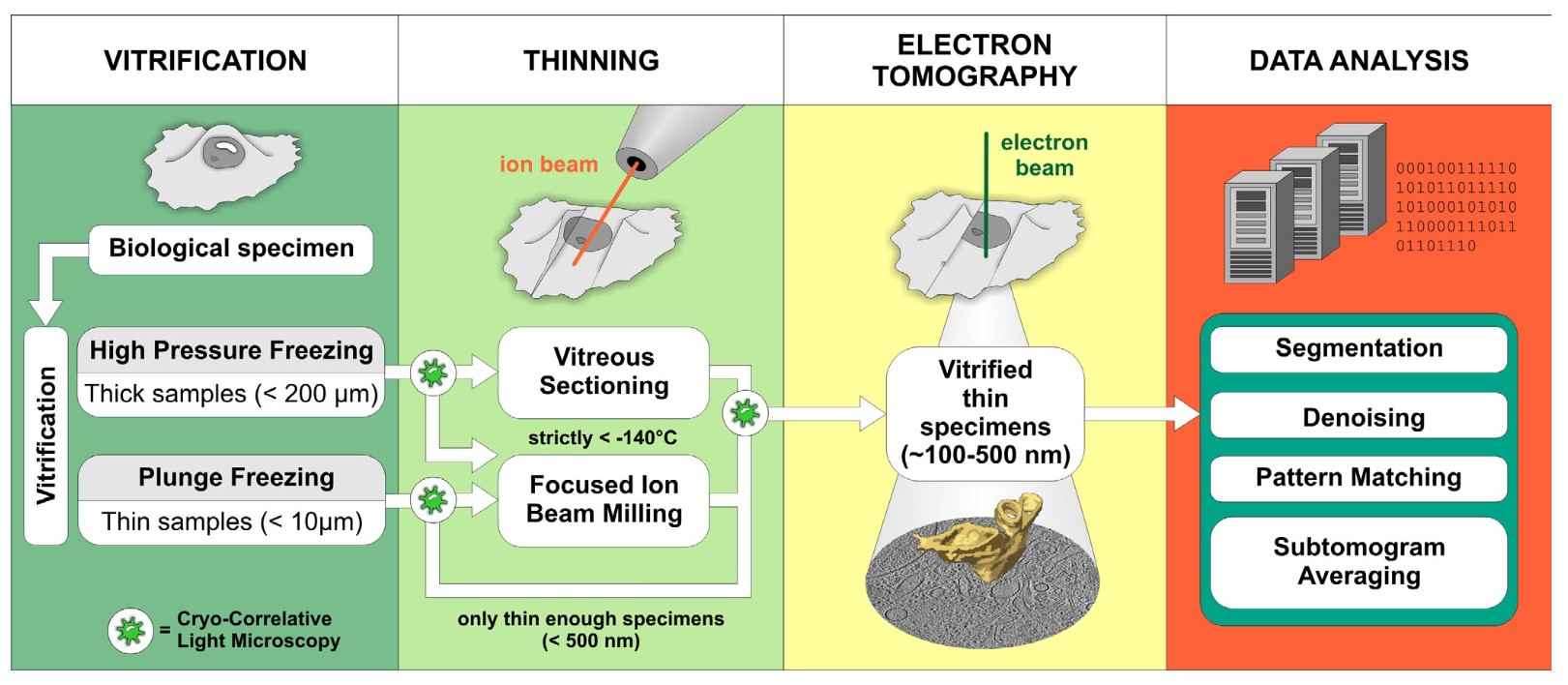 Figure 1. Schematic representation of the cryo-ET workflow (Lučić et al., 2013).
Figure 1. Schematic representation of the cryo-ET workflow (Lučić et al., 2013).
Advantages of Cryo-ET
Cryo-ET offers several significant advantages for structural biology:
Table 1. Advantages of Cryo-ET technique.
| Native-State Imaging | Similar to cryo-EM, cryo-ET preserves biological samples in a near-native state by freezing them rapidly. This prevents structural alterations caused by dehydration or fixation. |
| 3D Visualization | Cryo-ET provides a true 3D view of biological structures, which is critical for understanding spatial relationships between components within cells or large macromolecular assemblies. |
| In Situ Studies | Cryo-ET can be used to study macromolecular complexes directly within cells, providing insights into how biological structures are organized in their natural cellular environments. |
Limitations of Cryo-ET
Despite these advantages, cryo-ET has several limitations:
Table 2. Limitations of Cryo-ET technique.
| Radiation Damage | Like all electron microscopy techniques, cryo-ET exposes samples to radiation damage, which limits the amount of information that can be collected from a single sample. |
| Lower Resolution | Compared to single-particle cryo-EM, cryo-ET generally achieves lower resolution, particularly for large, complex samples. However, recent advancements in detectors and data processing are improving this aspect. |
| Complex Data Acquisition | The need to capture multiple images at different angles makes cryo-ET a time-consuming process. Additionally, the computational requirements for 3D reconstruction are substantial. |
| Sample Thickness | While cryo-ET can visualize larger samples than cryo-EM, it still faces limitations with very thick samples, requiring techniques like FIB milling to reduce sample thickness for optimal imaging. |
Applications of Cryo-ET
Cryo-ET has become a powerful tool for studying biological structures in situ, providing valuable insights into how macromolecules and organelles function within their native cellular environments. Key applications of cryo-ET include:
Cellular Architecture: Cryo-ET has been extensively used to study the organization of cells and their organelles. By imaging whole cells or large sections of cells, cryo-ET provides unique insights into cellular architecture, such as the organization of membranes, ribosomes, cytoskeletal elements, and other macromolecular complexes.
Macromolecular Complexes: Cryo-ET allows researchers to study large macromolecular assemblies, such as ribosomes, proteasomes, and viral capsids, directly within cells. This provides critical information about the spatial organization and functional dynamics of these complexes.
Membrane Proteins: Cryo-ET is particularly valuable for studying membrane proteins, which are difficult to crystallize. Membrane proteins can be visualized within their native lipid bilayers, offering a more accurate representation of their structure and function.
Pathogen-Host Interactions: Cryo-ET has been employed to study how viruses and other pathogens interact with their host cells. For example, cryo-ET has provided detailed images of how viruses enter cells, replicate, and assemble viral particles.
Structural Virology: Cryo-ET has advanced the understanding of viral structure, including the architecture of viral envelopes and capsids, and how these structures change during viral infection and replication.
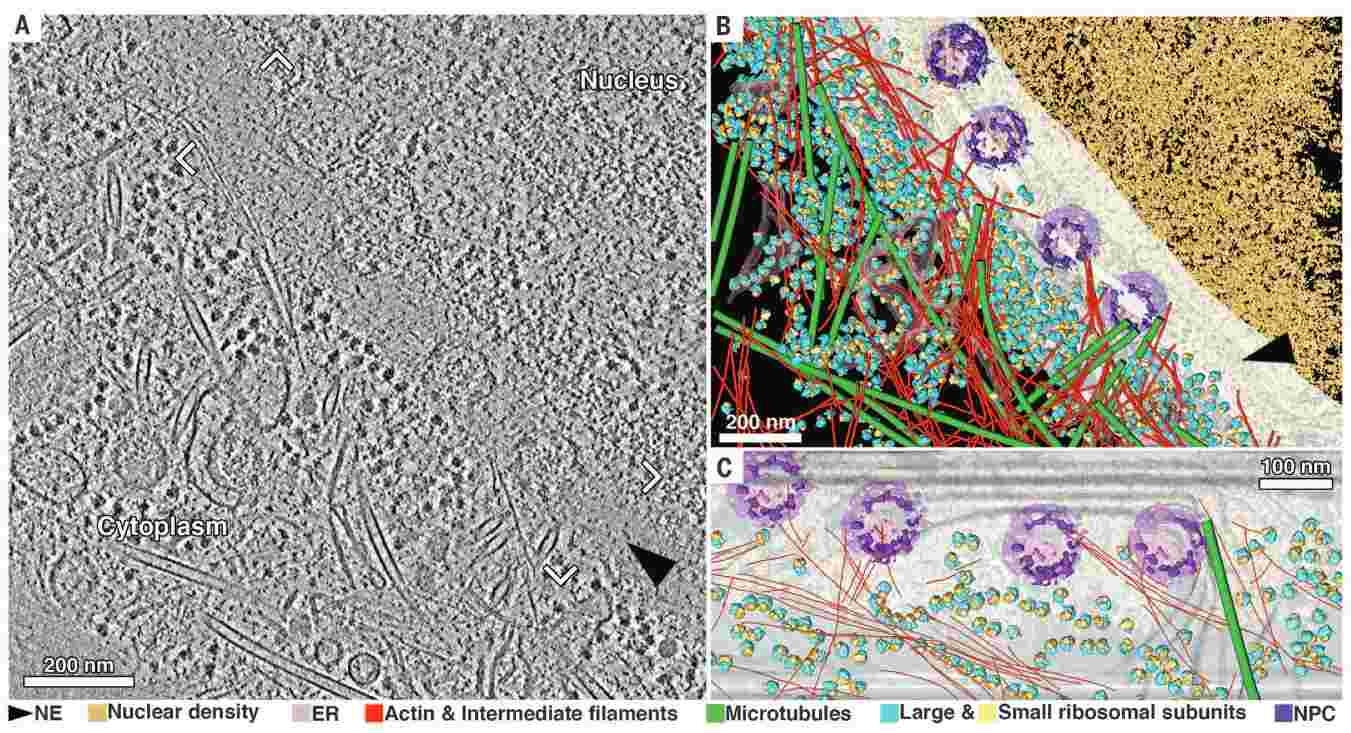 Figure 2. The nuclear periphery of a HeLa cell revealed by cryo-ET. (A) Tomographic slice with 8.4-nm thickness of an interphase HeLa cell thinned by cryo-FIB. (B) Annotated view of the tomographic data. Color labels are defined for each structure in (B) and (C). (C) Cross-section view of the segmentation in the vicinity of the nuclear envelope [frame in (A)]. NE: nuclear envelope; ER: endoplasmic reticulum; NPC: nuclear pore complex (Mahamid et al., 2016).
Figure 2. The nuclear periphery of a HeLa cell revealed by cryo-ET. (A) Tomographic slice with 8.4-nm thickness of an interphase HeLa cell thinned by cryo-FIB. (B) Annotated view of the tomographic data. Color labels are defined for each structure in (B) and (C). (C) Cross-section view of the segmentation in the vicinity of the nuclear envelope [frame in (A)]. NE: nuclear envelope; ER: endoplasmic reticulum; NPC: nuclear pore complex (Mahamid et al., 2016).
In conclusion, Cryo-Electron Tomography (Cryo-ET) is a powerful technique for visualizing biological structures in three dimensions, providing critical insights into the spatial organization of cells and macromolecules in their native states. With its ability to study complex, dynamic systems in situ, cryo-ET offers a unique window into cellular architecture and function. Despite its challenges, including radiation damage and lower resolution compared to single-particle cryo-EM, cryo-ET's applications in structural biology are vast, ranging from the study of cellular architecture and macromolecular complexes to membrane proteins and pathogen-host interactions. As technological advancements continue, cryo-ET is poised to become even more integral to the field of structural biology.
Creative Biostructure offers specialized Cryo-ET services, enabling you to explore the intricate architecture of cells and organelles with unparalleled detail. Whether you're studying molecular complexes or cellular structures, our advanced technology and expert team are here to support your research. Contact us today to discover how our cryo-ET services can accelerate your structural biology projects and lead to new scientific breakthroughs.
References
- Koning, R. I., & Koster, A. J. (2009). Cryo-electron tomography in biology and medicine. Annals of Anatomy, 191(5), 427–445.
- Lučić, V., Rigort, A., & Baumeister, W. (2013). Cryo-electron tomography: The challenge of doing structural biology in situ. Journal of Cell Biology, 202(3), 407–419.
- Mahamid, J., Pfeffer, S., Schaffer, M., Villa, E., Danev, R., Cuellar, L. K., Förster, F., Hyman, A. A., Plitzko, J. M., & Baumeister, W. (2016). Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science (New York, N.Y.), 351(6276), 969–972.
- Milne, J. L. S., & Subramaniam, S. (2009). Cryo-electron tomography of bacteria: Progress, challenges and future prospects. Nature Reviews Microbiology, 7(9), 666–675.
