Amino Acid Properties and Structure
Amino acids are building blocks of proteins connected by a specific type of covalent linkage. Amino acids are vital to life because the proteins consisting of them are involved in almost all cellular functions. Some proteins act as enzymes, some as antibodies, and others provide structural support. In addition to serving as building blocks for peptides and proteins, amino acids (either directly or in their modified form) are involved in many metabolic functions. They include neurotransmitter functions as well as precursors for the synthesis of non-protein nitrogen-containing metabolites. Although hundreds of amino acids have been found in nature, proteins are made up of 20 amino acids.
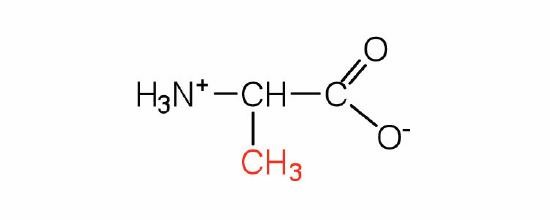
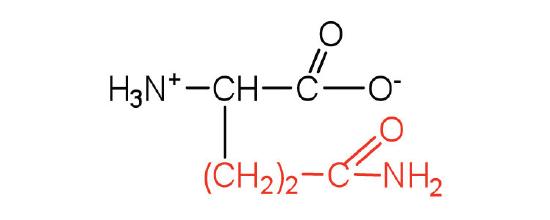
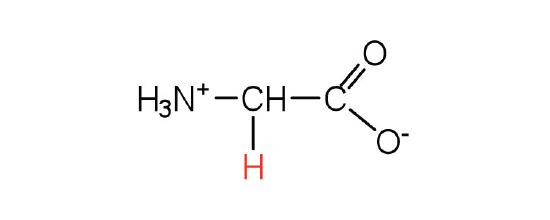
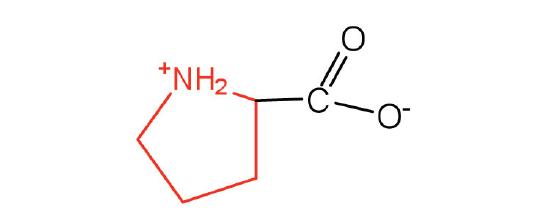
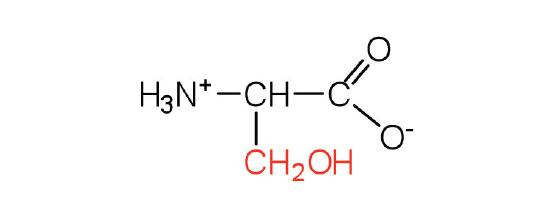
The basic structure of an amino acid consists of an amino group (-NH2), a carboxylic acid group (-COOH), a hydrogen atom, and a unique side chain termed "R-group". Each molecule contains a central carbon atom, known as α-carbon, to which both the amino and carboxylic acid groups are attached. The remaining two bonds of the α-carbon atom are usually satisfied by a hydrogen atom and an R group. Thus, the α-carbon atom is attached to four substituents. Amino acids differ from each other in the specific chemical properties and structure of the R groups.
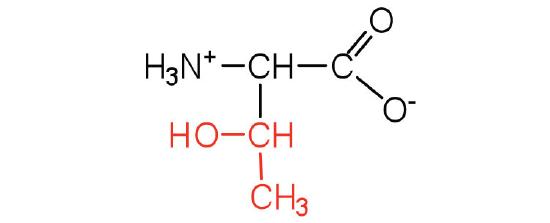
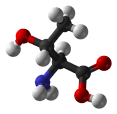
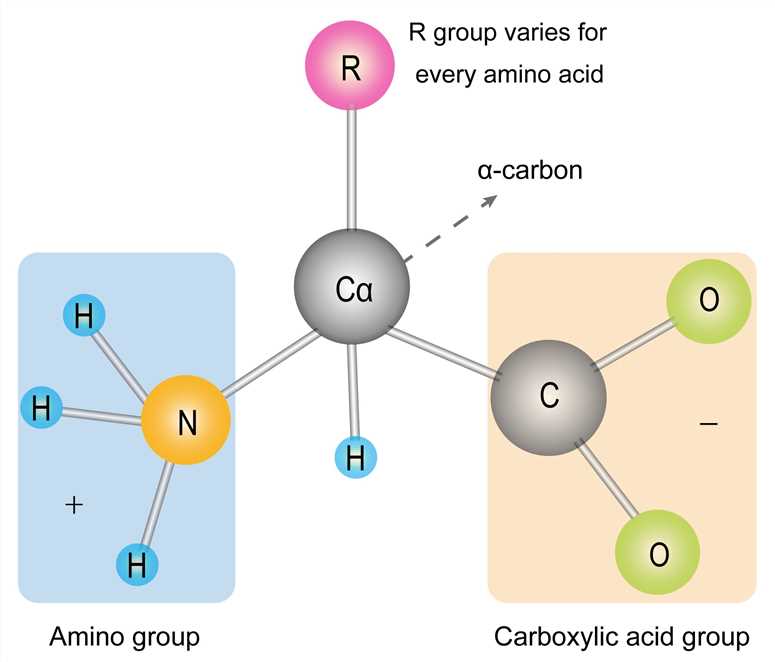 Figure 1. The amino acid structure diagram.
Figure 1. The amino acid structure diagram.
| Name/ Code | M.W. | pI | pKa, NH2 | pKa, COOH | pK (R) | Solubility | Formula/ Structure | 3D Structure |
|---|---|---|---|---|---|---|---|---|
| Alanine/ Ala (A) | 89.10 | 6.00 | 9.87 | 2.35 | - | 15.8 | C3H7NO2
|
|
| Arginine/ Arg (R) | 174.20 | 10.76 | 9.09 | 2.18 | 13.2 | 71.8 | C6H14N4O2
|
|
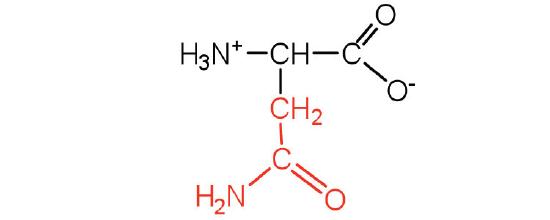
| Asparagine/ Asn (N) | 132.12 | 5.41 | 8.8 | 2.02 | - | 2.4 | C4H8N2O3
|
|
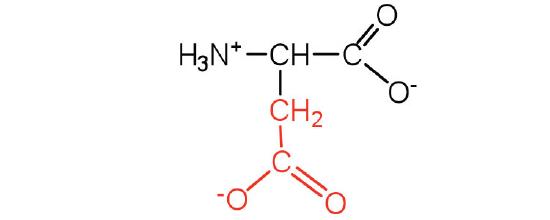
| Aspartic Acid/ Asp (D) | 133.11 | 2.77 | 9.6 | 1.88 | 3.65 | 0.42 | C4H7NO4
|
|
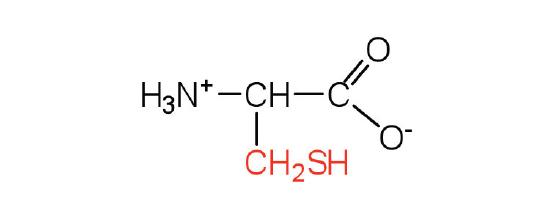
| Cysteine/ Cys (C) | 121.16 | 5.07 | 10.78 | 1.71 | 8.33 | freely | C3H7NO2S
|
|
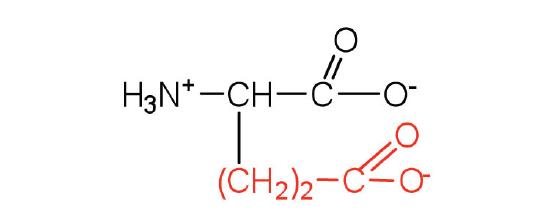
| Glutamic Acid/ Glu (E) | 147.13 | 3.22 | 9.67 | 2.19 | 4.25 | 0.72 | C5H9NO4
|
|
| Glutamine/ Gln (Q) | 146.15 | 5.65 | 9.13 | 2.17 | - | 2.6 | C5H10N2O3
|
|
| Glycine/ Gly (G) | 75.07 | 5.97 | 9.6 | 2.34 | - | 22.5 | C2H5NO2
|
|
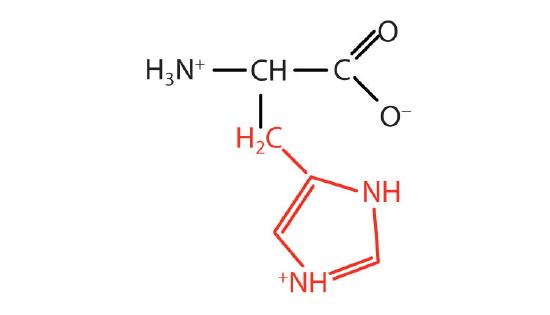
| Histidine/ His (H) | 155.16 | 7.59 | 8.97 | 1.78 | 6 | 4.19 | C6H9N3O2
|
|
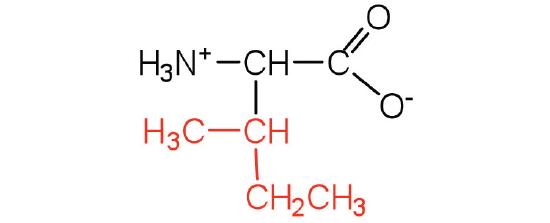
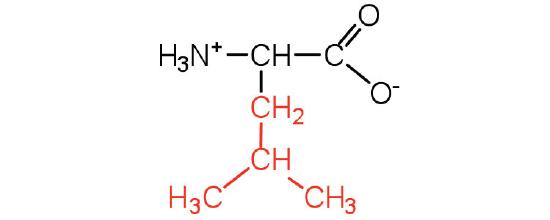
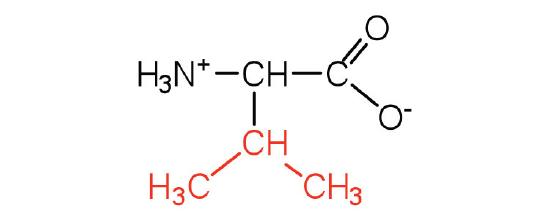
| Isoleucine/ Ile (I) | 131.18 | 6.02 | 9.76 | 2.32 | - | 3.36 | C6H13NO2
|
|
| Leucine/ Leu (L) | 131.18 | 5.98 | 9.6 | 2.36 | - | 2.37 | C6H13NO2
|
|
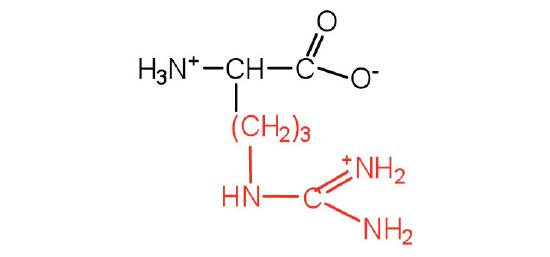
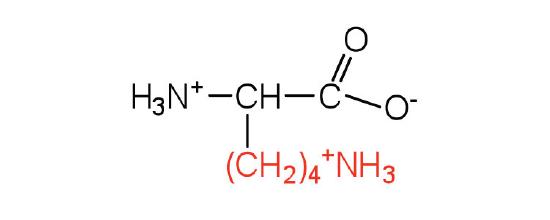
| Lysine/ Lys (K) | 146.19 | 9.74 | 10.28 | 8.9 | 2.2 | - | C6H14N2O2
|
|
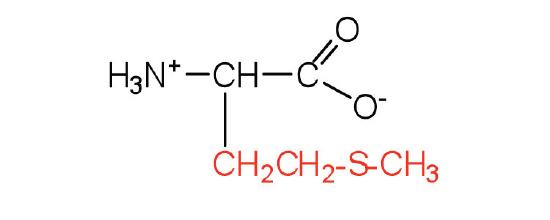
| Methionine/ Met (M) | 149.21 | 5.74 | 9.21 | 2.28 | - | 5.14 | C5H11NO2S
|
|
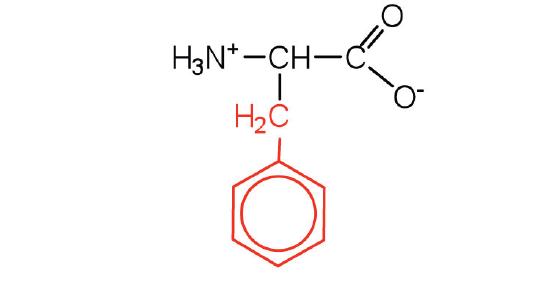
| Phenylalanine/ Phe (F) | 165.19 | 5.48 | 9.24 | 2.58 | - | 2.7 | C9H11NO2
|
|
| Proline/ Pro (P) | 115.13 | 6.30 | 10.6 | 1.99 | - | 1.54 | C5H9NO2
|
|
| Serine/ Ser (S) | 105.09 | 5.68 | 9.15 | 2.21 | - | 36.2 | C3H7NO3
|
|
| Threonine/ Thr (T) | 119.12 | 5.60 | 9.12 | 2.15 | - | freely | C4H9NO3
|
|
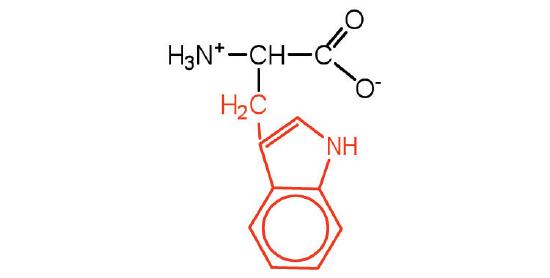
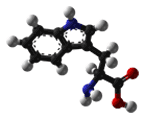
| Tryptophan/ Trp (W) | 204.23 | 5.89 | 9.39 | 2.38 | - | 1.06 | C11H12N2O2
|
|
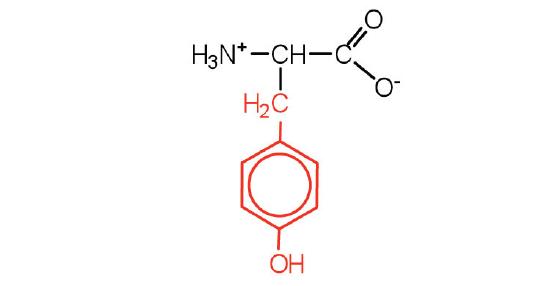
| Tyrosine/ Tyr (Y) | 181.19 | 5.66 | 9.11 | 2.2 | 10.1 | 0.038 | C9H11NO3
|
|
| Valine/ Val (V) | 117.15 | 5.96 | 9.72 | 2.29 | - | 5.6 | C5H11NO2
|
|
Table 1. Amino acid properties and structure.
Amino acid classification
Based on the properties of the R group, the 20 common amino acids are classified as follows:
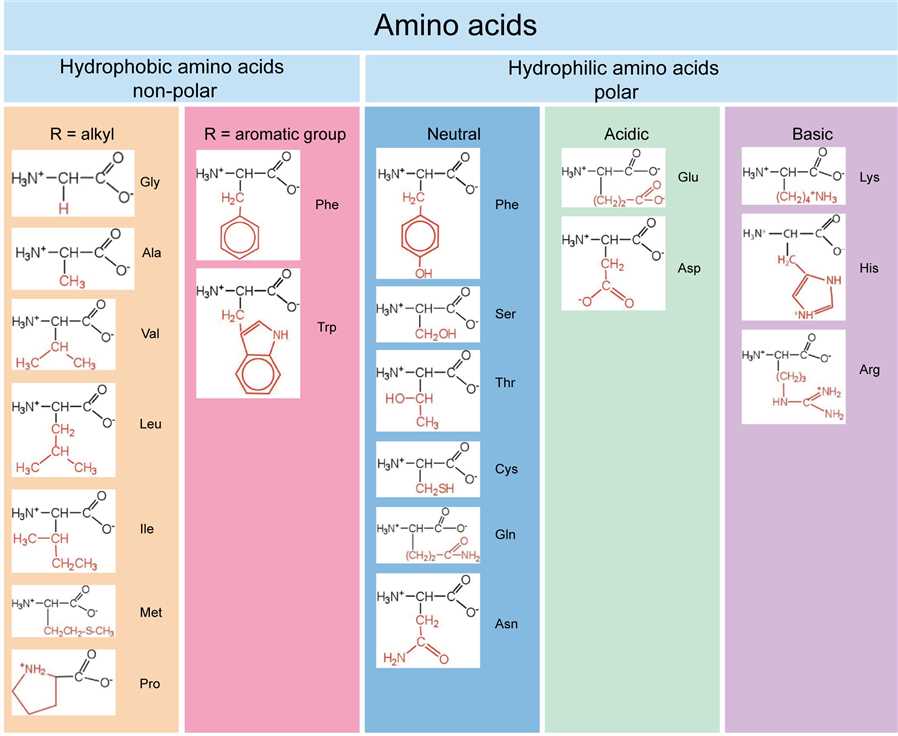 Figure 2. Amino acid classification.
Figure 2. Amino acid classification.
How are amino acids joined together?
The amino acid can be connected by a condensation reaction in which the nucleophilic addition-elimination reaction between the amino group of one amino acid and the carboxyl group of another amino acid forms a covalent bond (peptide bond), and releases one molecule of water as a by-product. And the peptide bond is essentially an amide bond.
Amino acids linked by a series of peptide bonds constitute a peptide chain, each amino acid of which is referred to as an amino acid residue. Polymers composed of less than 50 amino acid residues are termed oligopeptides, while larger polymers (more than 50 amino acid residues) are known as polypeptides. Thus, a protein molecule is a polypeptide chain composed of many amino acid residues, with each residue linked to the next residue by a peptide bond.
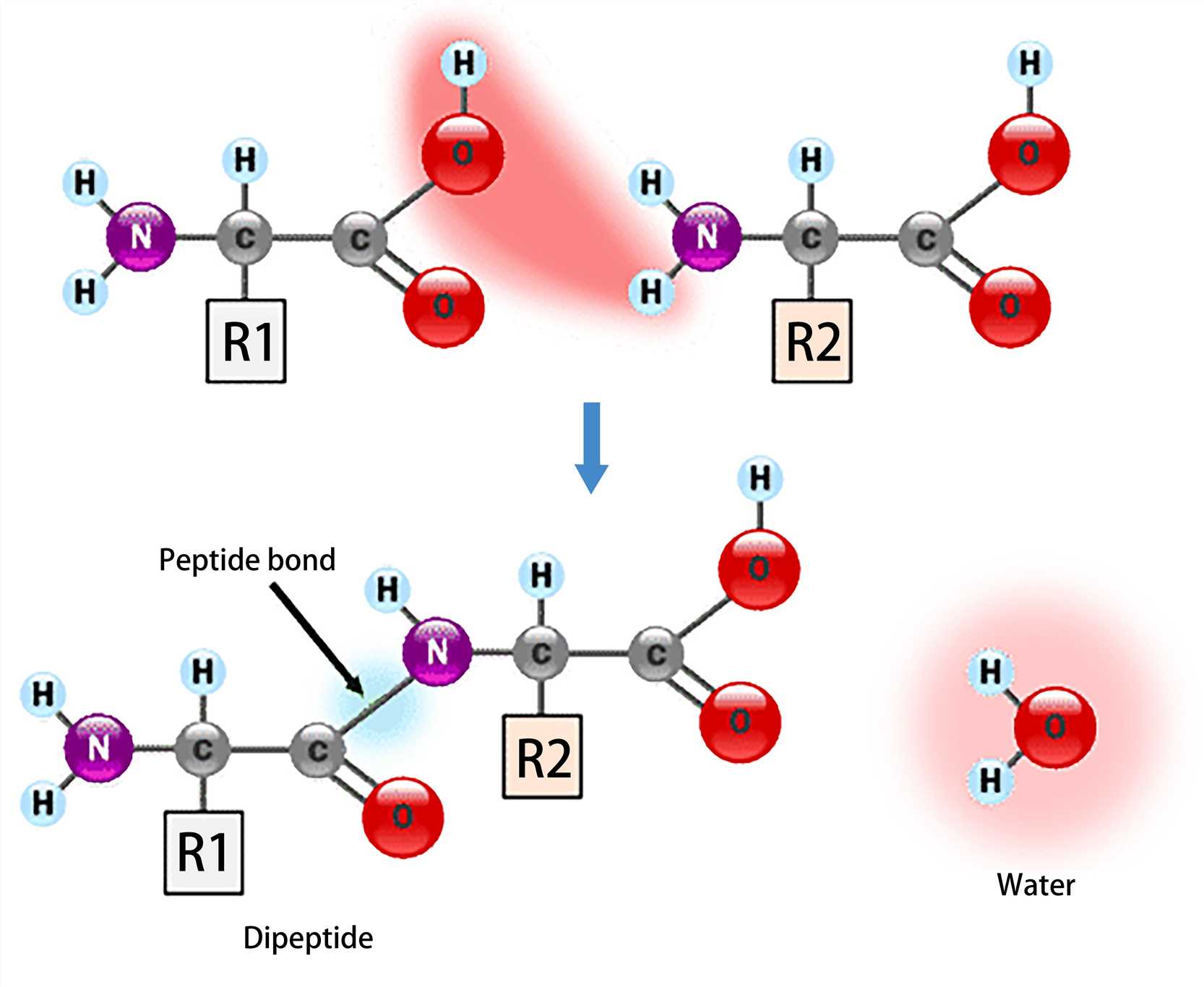 Figure 3. Peptide bond formation.
Figure 3. Peptide bond formation.
Creative Biostructure is specialized in the field of structural biology, and we provide contract services for the structural analysis of proteins of your interest.