Virus-like Particle Quantification Services
Creative Biostructure is a leading provider of virus-like particle (VLP) quantification services, utilizing state-of-the-art technology to deliver comprehensive analyses. VLPs serve as powerful tools for antigen presentation, drug delivery, vaccine development, and nanotechnology applications. VLP quantification involves the precise measurement and characterization of VLPs in terms of their concentration, size distribution, and surface protein content. With our expertise and cutting-edge technology, we offer a range of advanced methods to help you ensure the quality of your VLP samples and support your research efforts.
Why Conduct Virus-like Particle Quantification?
Accurate quantification of VLPs is critical because their structure and function differ from traditional single-protein therapeutics. Given their similarity to extracellular vesicles (EVs), which can sometimes co-purify with VLPs, high-resolution techniques are required to distinguish and quantify them accurately. Traditional methods do not provide the resolution needed to differentiate between such particles, which is why cutting-edge technologies like super-resolution fluorescence microscopy (SRFM), nanoparticle tracking analysis (NTA), and flow virometry (FVM) are used. These technologies allow for detailed and precise VLP quantification, ensuring their structural integrity, size, and protein composition.
VLP quantification is essential for a wide variety of applications, including:
- Vaccine Development: Accurate VLP characterization ensures the correct dose and structure of the antigen, which is crucial for eliciting the desired immune response.
- Drug Delivery: In drug delivery systems, precise quantification of VLPs helps optimize dosage forms and ensures consistency in drug release profiles.
- Nanotechnology: VLPs are used as nanocarriers, and their accurate quantification ensures they meet the desired specifications for research and development in nanomedicine.
In all these applications, VLP quantification helps maintain product quality, safety, and efficacy.
Our Techniques for Virus-like Particle Quantification
We offer VLP quantification services using state-of-the-art technology to accurately determine VLP concentrations, size distributions, and other key parameters. Our services are mainly provided using the following techniques:
Super-Resolution Fluorescence Microscopy (SRFM)
SRFM allows imaging and analysis of VLPs at resolutions beyond conventional microscopes. By utilizing advanced fluorescence microscopy, we can image and identify target membrane proteins with superior spatial resolution. This technique provides valuable insights into VLP morphology, size, structural integrity, and the localization of membrane proteins.
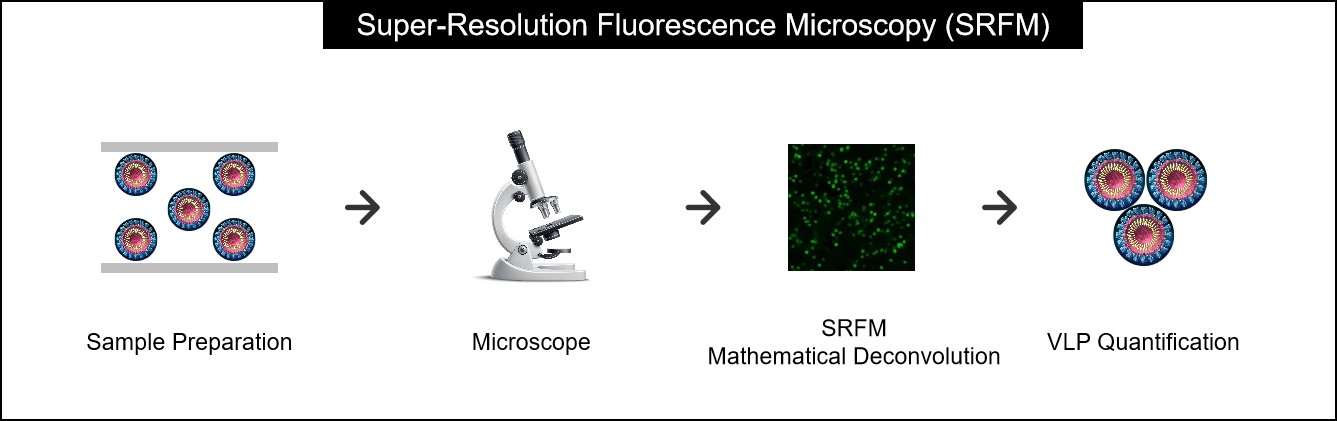 Figure 1. Sample preparation and analysis workflow for SRFM. (Creative Biostructure)
Figure 1. Sample preparation and analysis workflow for SRFM. (Creative Biostructure)
Nanoparticle Tracking Analysis (NTA)
NTA is a powerful technique for measuring particle size distribution and concentration in liquid samples. Our NTA-based service tracks individual VLPs in real-time. Using specific fluorescent probes or antibodies, we detect and quantify desired membrane proteins, providing important information about their abundance and distribution. The service provides valuable information on VLP stability, polydispersity and sample purity.
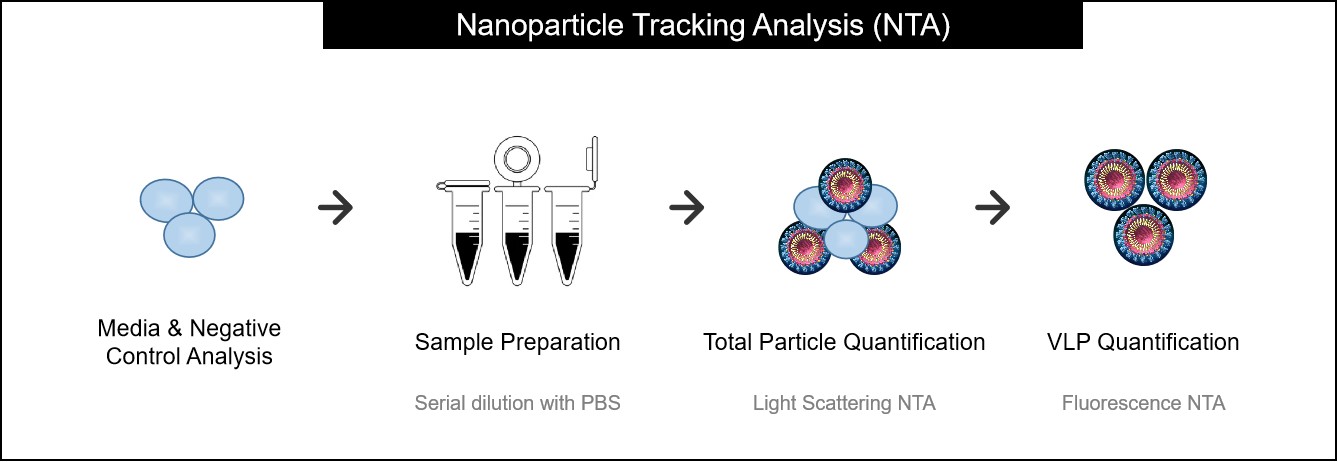 Figure 2. Sample preparation and analysis workflow for NTA. (Creative Biostructure)
Figure 2. Sample preparation and analysis workflow for NTA. (Creative Biostructure)
Flow Virometry
Flow Virometry combines flow cytometry and fluorescence detection to analyze individual VLPs in a high-throughput manner. Our service accurately measures VLP concentration, size, and surface properties. Using fluorescently labeled antibodies or probes, we assess specific membrane proteins, providing detailed analysis of protein expression levels. This technique helps evaluate VLP heterogeneity and is valuable for assessing VLP-based vaccine candidates.
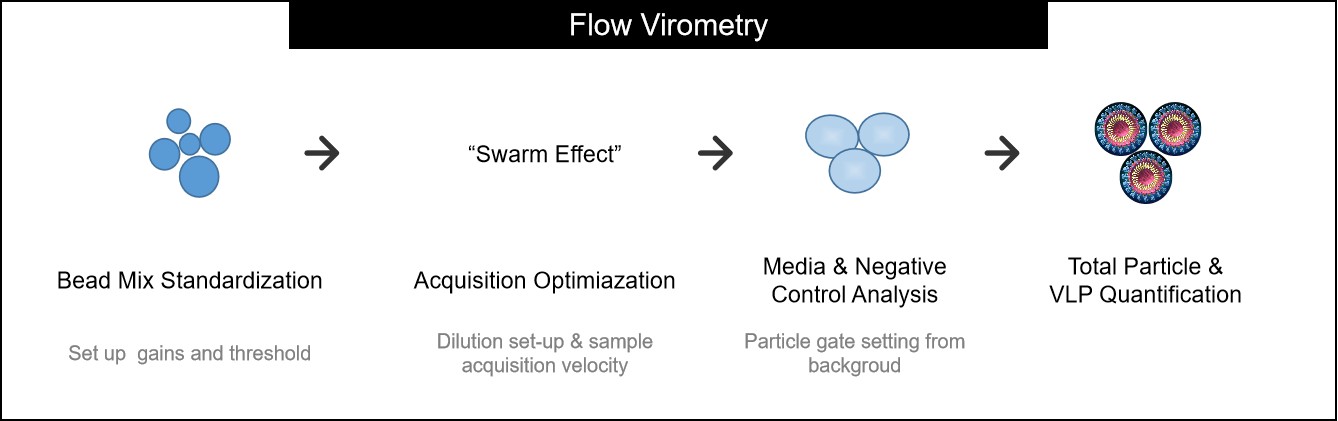 Figure 3. Sample preparation and analysis workflow for flow virometry. (Creative Biostructure)
Figure 3. Sample preparation and analysis workflow for flow virometry. (Creative Biostructure)
Workflow for Our VLP Quantification Services
At Creative Biostructure, our VLP quantification services follow a systematic workflow to ensure reliable and accurate results.
- Sample Preparation: VLPs are extracted and purified from samples using optimized protocols to minimize contaminants like extracellular vesicles.
- Quantification Method Selection: Depending on the specific needs of the project, one or more advanced techniques are chosen.
- Super-Resolution Fluorescence Microscopy (SRFM): Allows high-resolution imaging beyond the diffraction limit of traditional microscopes to assess the morphology and spatial distribution of membrane proteins.
- Nanoparticle Tracking Analysis (NTA): Measures particle size and concentration in real-time, providing insight into sample purity and polydispersity.
- Flow Virometry: Combines flow cytometry and fluorescence detection for high-throughput analysis of individual VLPs, offering insights into surface properties and protein expression levels.
- Data Acquisition and Analysis: Data is collected using state-of-the-art instruments and analyzed to provide key insights into VLP size distribution, concentration, and structural integrity.
- Reporting: A comprehensive report is generated, detailing the VLP quantification results, including graphical representations of size distribution, protein content, and any identified impurities.
- Consultation and Support: Our team can provide detailed consultations on the results, offering recommendations for further steps in research or product development.
Why Choose Creative Biostructure?
- Cutting-Edge Technologies: We utilize advanced techniques such as SRFM, NTA, and flow virometry to provide comprehensive VLP quantification services.
- Expertise and Experience: Our team of experts has extensive experience in VLP research and analysis. We follow standardized protocols and employ rigorous quality control measures to ensure accurate and reliable results.
- Tailored Solutions: Our services can be customized to meet your specific requirements, ensuring that you receive tailored solutions that address your research goals.
- Timely Delivery and Confidentiality: We prioritize delivering high-quality data within the agreed-upon timeframe. We maintain strict confidentiality and data security protocols to protect the privacy of our clients and their research.
- Comprehensive Service Portfolio: In addition to VLP quantification, we offer a wide range of related services, including VLP production, purification, characterization, and functional analysis.
Frequently Asked Questions
-
How do I choose the right quantification method for my VLP sample?
The choice depends on your specific research needs. NTA is suitable for real-time tracking and concentration measurements, while SRFM offers high-resolution imaging of structural details. Flow virometry is ideal for high-throughput analysis. Our experts can guide you based on your project's goals.
-
Can you quantify VLPs in the presence of extracellular vesicles?
Yes, our advanced technologies, such as NTA and flow virometry, can distinguish between VLPs and extracellular vesicles, allowing for accurate quantification of each particle type.
-
How long does it take to receive results from VLP quantification?
Turnaround time varies depending on the complexity of the analysis and the sample type. Most projects are completed within 1-2 weeks. We also offer expedited services for urgent requests.
To learn more about our VLP quantification services or to discuss your specific project requirements, please contact our team. We look forward to working with you to advance your research.
