Liposome Analysis and Characterization
Creative Biostructure utilizes a wide range of techniques for liposome analysis based on our comprehensive Liposome Platform. Determination of various liposome properties are often desired during development of a specific product or process.
The classes of phospholipid present in a particular phospholipid fraction will influence the characteristics of any liposomes produced from that fraction. Stability Analysis
There are two main areas in liposome stability, which are stability during storage and/or processing and stability in the body. For pharmaceutical applications, the latter is extremely important, as the release of drugs in the blood system needs to be very well defined.
The size, shape, entrapped volume and lamellarity of a liposome dispersion are all interdependent. Liposome lamellarity has a significant effect on encapsulation efficiency and the rate of diffusion of encapsulated contents out of the interior spaces of the liposome. Size Analysis
It is usually necessary to utilize electron microscopy to achieve definite confirmation that liposomes are present in a sample because of their small size, but the size distribution is also an helpful indirect method to detect the presence of liposome. Permeability Analysis
The relative permeability will vary depending on the molecule, the environmental conditions, the composition of the membrane and inter/extra liposomal fluid, and the processing/storage history of the solution.
Measurements such as the entrapped aqueous volume per unit of phospholipid provides useful information to predict the entrapment efficiency of the population. The concentration of the phospholipids and the encapsulated contents are also crucial characteristics of liposomal systems and are required to calculate liposomal yields, costs, and optimise processes. Creative Biostructure employs various methods to characterize the liposome solution and the drug-loaded liposomes.
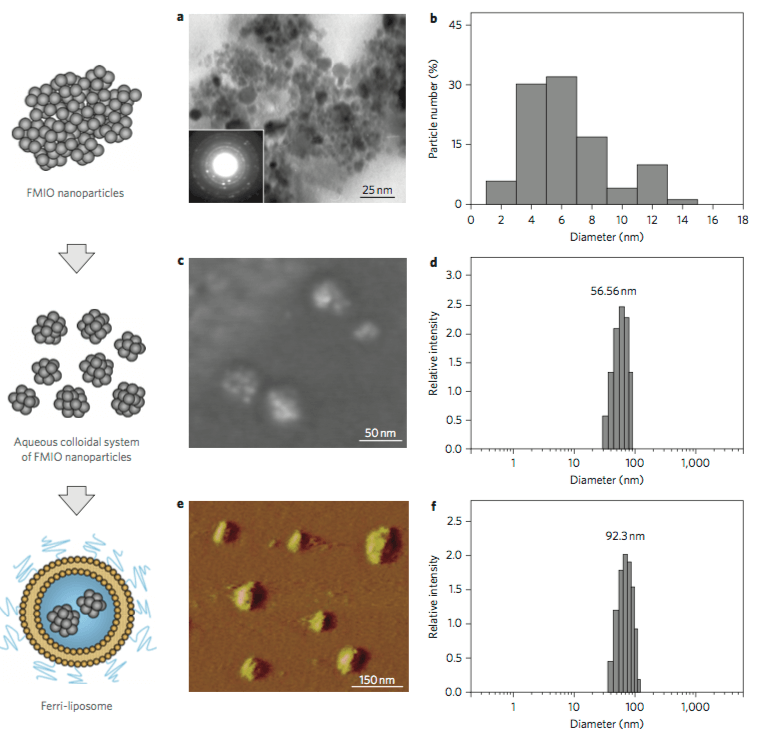 Figure 1. Characterization of the magnetic nanocarrier system. The liposome size was characterized by DLS. (G. Mikhaylov,
et al
., 2011)
Figure 1. Characterization of the magnetic nanocarrier system. The liposome size was characterized by DLS. (G. Mikhaylov,
et al
., 2011)
Our analysis and characterization services include but are not limited to:
- Nano Flow Cytometry Liposome Analysis
- Liposome Spatial Omics Service
- Liposome Lamellarity Analysis
- Liposome Lipid Content Analysis
- Liposome Size Analysis
- Liposome Stability Analysis
- Liposome Permeability Analysis
- Liposome Encapsulation Efficiency Determination
- Liposome Visualization
- Liposome Zeta Potential Determination
- Liposome Turbidity Measurement
- Liposome Penetration and Permeation Test
- Liposome Drug Release Kinetics
-
Liposome Localization Service
Identifying appropriate methods for tracing and imaging liposomes is advantageous for studying drug delivery and release processes, enabling effective and stable tracking of liposomes.
Liposome Analysis Project Process
Besides Liposome Analysis and Characterization, Creative Biostructure is more than happy to provide you other Liposome Technology based products and services. Please feel free to contact us for a detailed quote.
Ordering Process
References
- Y. Yoon, et al . (2014). Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics , 4(11): 1133-1144.
- G. Mikhaylov, et al . (2011). Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nature Nanotechnology , 6(9): 592-604.
- A. Akbarzadeh, et al . (2013). Liposome: classification, preparation, and applications. Nanoscale Research Letters, 8: 102.

