Liposome Encapsulation Efficiency Determination
Creative Biostructure has developed the advanced Mempro™ Liposome technology to support the research of drug delivery. Not only do we have excellent technique for liposome design and manufacture, but we have also possessed a comprehensive set of equipment for liposome analysis. We can offer accurate analysis service for encapsulation efficiency determination.
Why encapsulation efficiency is important to liposome?
The encapsulation efficiency (EE%) is the percentage of drug that is successfully entrapped into liposomes. It is defined by the concentration of the incorporated material detected in the formulation over the initial concentration used to make the formulation. Encapsulation efficiency is one of the most important parameters for liposome formulation as it directly reflects the concentration of active ingredients (drugs, proteins, antimicrobial agents, etc.). Factors affecting the encapsulation efficiency of the drug depend on properties of both the liposome and encapsulated drugs. Concerning the encapsulated drugs, the encapsulation efficiency is affected by their hydrophilicity or lipophilicity. A number of liposome properties, such as aqueous volume, membrane rigidity, surface area and preparation methods are reported to have influence on the encapsulation efficiency.
Liposome encapsulation efficiency analysis methods
In order to calculate encapsulation efficacy, the first step is to separate non-entrapped drug from drug-loaded liposomes. Method used for separation should be fast and simple, and excessive sample dilution and the loss of drug from liposome inside should be avoided. Centrifuge and exclusion column have been used widely for separation.
The concentration of drugs can be detected using selected method according to their properties. Usually, high-performance liquid chromatography (HPLC) is the simplest way to get accurate results for the amount of total drugs and unencapsulated drugs respectively. As for siRNA drugs, agarose gel electrophoresis method can be used to monitor the amount of siRNA. For protein-carrier, the concentration of protein (free drug) can be detected by UV-vis absorbance or dye-binding assays. Besides, circular dichroism (CD) spectroscopy is a simple and convenient method to determine the composition of various protein-carrier conjugates, in the meantime avoiding overlap of UV signals.
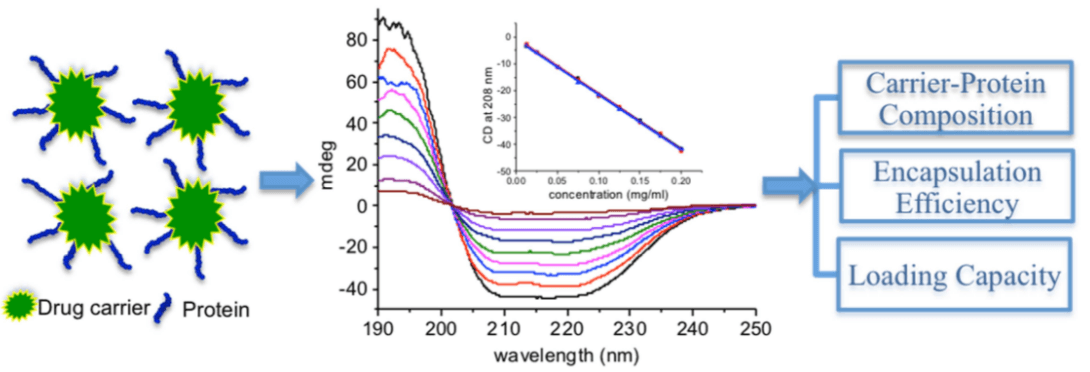 Figure 1: Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy.(Peng Z, et al. Anal Chim Acta. 2016)
Figure 1: Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy.(Peng Z, et al. Anal Chim Acta. 2016)
Creative Biostructure offers reliable service for liposome characterization. We are specialized to design and develop custom analytical method suitable for the characterization (e.g., encapsulation efficiency) of specific samples. Please contact us to share more about your research and get a detailed quote.
Ordering Process
References
- ZhiliPeng, et al. (2016). Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy. Analytica Chimica Acta, 937: 113-118.
- Yamamoto E, et al. (2018). A simple and rapid measurement method of encapsulation efficiency of doxorubicin loaded liposomes by direct injection of the liposomal suspension to liquid chromatography. Int J Pharm, 536:21-28.
- J. F. Boelter and A Brandelli. (2016). Innovative bionanocomposite films of edible proteins containing liposome-encapsulated nisin and halloysite nanoclay. Colloids and Surfaces B: Biointerfaces, 145: 740-747.
