Plasma Membrane Protein Isolation
Plasma membrane has been proven to be more than a physical barrier between the cell and the outside environment. Being selectively permeable to ions and organic molecules, it controls the movement of substances in and out of cells. Besides, plasma membrane is also involved in many cellular processes, such as cell adhesion, ion conductivity and cell signaling. Studies on membrane proteins will further elucidate the important functions played by plasma membrane.
Isolating plasma membrane is an essential step in the study of membrane protein functions. Here at Creative Biostructure, several different approaches are utilized to efficiently isolate plasma membranes, based on the following principles:
1. Ionic interaction
The simplest method involves the use of cationic polylysine, which can increase cell adherence by interacting with negatively charged cells. Cells, in this case, are attached to polylysine-coated glass plates and then ruptured by hypotonic pressure. A plasma membrane fraction remains attached to the plates after washing away the intracellular organelles. Trypsin digestion then releases the glycoproteins from the plasma membrane, allowing them to be further analyzed. Glycoproteins released by trypsin digestion can be readily coupled with subsequent proteomic studies as well. This technique is therefore a relatively “clean” and scalable approach for plasma membrane isolation.
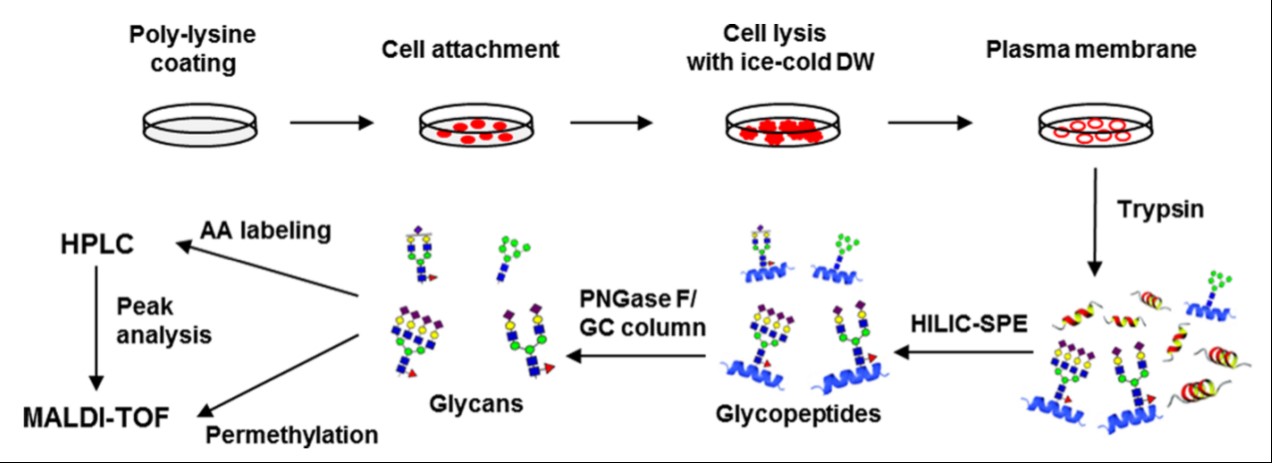 Figure 1. Overall strategy of a cell surface N-glycan analysis through the adhesion-based isolation of the plasma membrane.
Figure 1. Overall strategy of a cell surface N-glycan analysis through the adhesion-based isolation of the plasma membrane.
2. Carbohydrate-binding protein
Lectin, a carbohydrate-binding protein, is also commonly used to isolate the extensively glycosylated plasma membrane proteins. Specifically, we use Concanavalin A (ConA), a lectin with mannose specificity, for plasma membrane isolation. In this method, ConA is immobilized onto magnetic beads, and isolates plasma membranes from homogenized cell lysates. The plasma membrane proteins can then be solubilized from the beads by detergents and used for further studies.
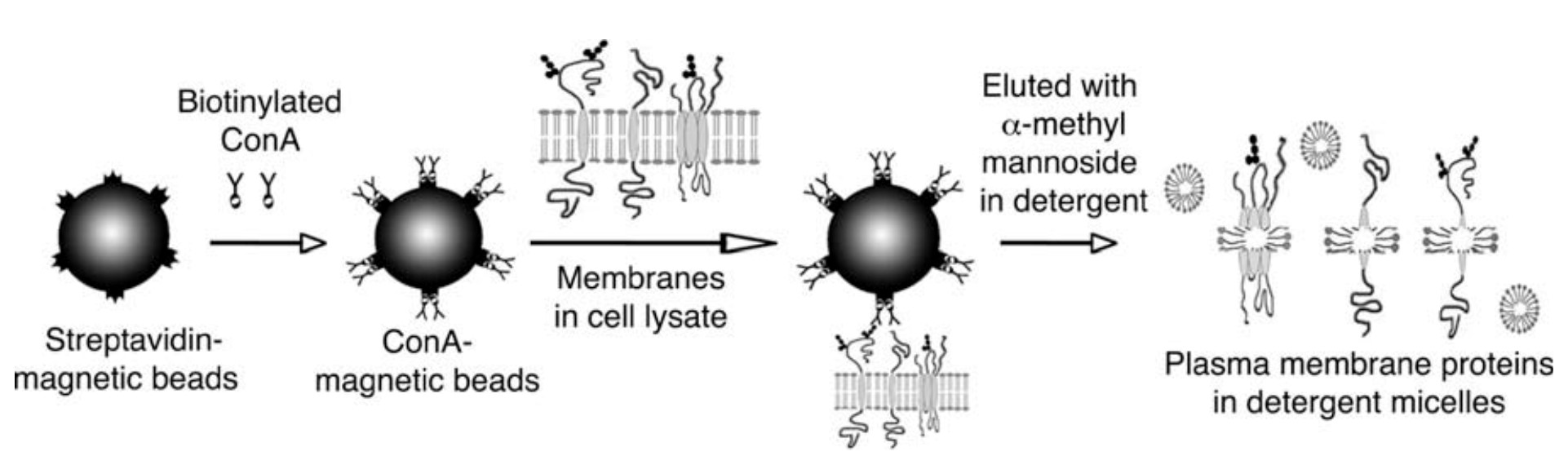 Figure 2. Schematic diagram of the plasma membrane isolation procedure using ConA.
Figure 2. Schematic diagram of the plasma membrane isolation procedure using ConA.
3. Ultracentrifugation
Creative Biostructure also uses ultracentrifugation as a conventional way to isolate plasma membrane by sucrose or iodixanol gradient methods. To isolate the plasma membrane, a low-speed centrifugation is normally performed first to remove the nuclei before the ultracentrifugation is applied. We also use Percoll gradient ultracentrifugation, which is proven to be a faster method; but removal of the Percoll is usually required before further plasma membrane studies.
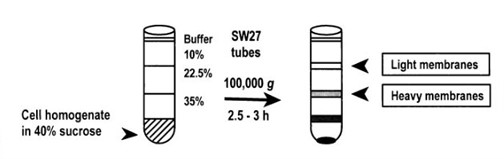 Figure 3. Subcellular fractionation of the cell homogenate from T lymphocyte cells produced plasma membrane fractions with specific gravity of 1.034 –1.09 g/ml (light membranes) and 1.09 –1.136 g/ml (heavy membranes).
Figure 3. Subcellular fractionation of the cell homogenate from T lymphocyte cells produced plasma membrane fractions with specific gravity of 1.034 –1.09 g/ml (light membranes) and 1.09 –1.136 g/ml (heavy membranes).
4. Extraction kits
Alternatively, easy-to-use extraction kits are commercially available for the fast isolation of membrane proteins using a bench top centrifuge, eliminating the need for ultracentrifugation. Along with the kits, optimized purification protocols are provided to offer consistent yield and high purity (> 90%). The whole purification procedure can be finished within 1 hour. Proteins purified using these kits can be directly used in enzyme assays, SDS-PAGE, Western blotting and other procedures.
Creative Biostructure offers high-quality services for MemPro™ plasma membrane isolation to facilitate the functional studies of the associated membrane proteins. Please see the list of membrane proteins provided by Creative Biostructure.
Please feel free to contact us for a detailed quote!
Ordering Process
References
- Lee YC, et al. (2015) “Lectin-magnetic beads for plasma membrane isolation”. Cold Spring Harb Protoc doi:10.1101/pdb. prot074427.
- Chekhun VF, et al. (2013) “Alteration in lipid composition of plasma membranes of sensitive and resistant Guerin carcinoma cells due to the action of free and liposomal form of cisplatin”. Exp Oncol 35(3):192-197.
- Mun JY, et al. (2013) “Efficient adhesion-based plasma membrane isolation for cell surface N-glycan analysis”. Anal Chem 85:7462−7470.
- Lee YC, et al. (2008) “One-step isolation of plasma membrane proteins using magnetic beads with immobilized concanavalin A”. Protein Expr Purif 62(2):223-229.
- Ilangumaran S, et al. (1999) “Microdomain-dependent regulation of lck and fyn protein-tyrosine kinases in T lymphocyte plasma membranes”. Molecular Biology of the Cell 10:891–905.
