Crystallization Chaperone Strategies
Membrane proteins, such as G protein-coupled receptors (GPCRs) and ion channels, are important drug targets. However, structure-based drug discovery (SBDD) is hampered by the lack of the corresponding 3D protein models, mostly due to the difficulty of crystallizing membrane proteins in their native state. One strategy to address this problem is to prepare the target membrane proteins in complex with covalent or non-covalent bound soluble protein chaperones that are highly intrinsic to crystallize.
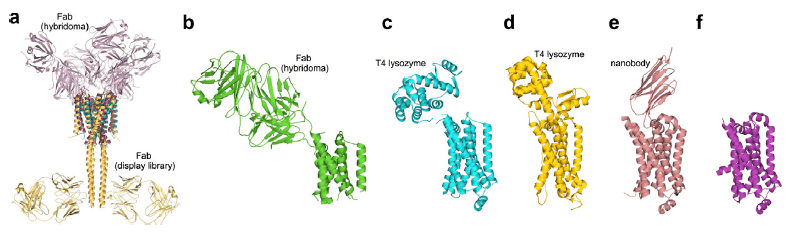 Figure 1. Comparison of selected membrane protein structures solved with and without chaperones.
Figure 1. Comparison of selected membrane protein structures solved with and without chaperones.
For covalent attachment of a chaperone to the membrane protein of interest, the DNA sequence encoding a highly flexible or non-conserved hydrophilic region is replaced with that of good solubility and high crystallization propensity. One the other hand, a non-covalent system is advantageous in that the chaperone and target membrane protein can be optimized independently for their behaviors in solution.
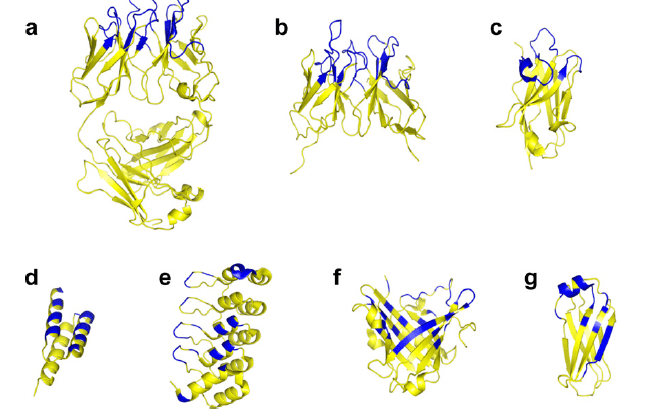 Figure 2. Chaperone platforms for membrane protein co-crystallization (yellow) with depicted relative sizes and binding surfaces (blue).
Figure 2. Chaperone platforms for membrane protein co-crystallization (yellow) with depicted relative sizes and binding surfaces (blue).
Strategies to improve diffraction quality of membrane protein crystals are multifaceted. Creative Biostructure provides different methods to optimize the chaperones for best crystallization of your target membrane proteins.
Ordering Process
