Liposome Platform
Liposomes are artificial spherical vesicles with enclosed phospholipid bilayer structures. They are now an established technique with considerable acceptance for the delivery of drugs, the assistance of membrane protein synthesis, and the application in nutrients, textiles dyes, and cosmetics. Creative Biostructure has developed top level liposome platform to support from manufacture to analysis for both industry and academia customers.
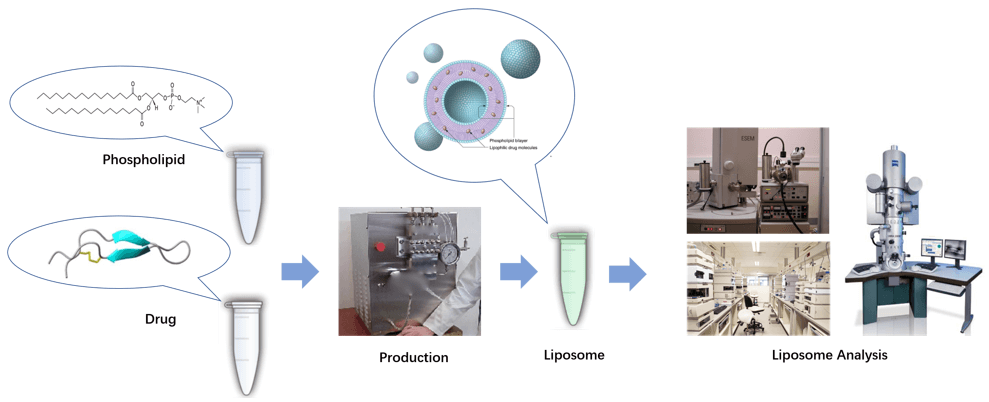 Figure 1. Workflow for liposome production and analysis.
Figure 1. Workflow for liposome production and analysis.
Platform Equipment
Our platform is equipped with high performance liposome homogenizer. Besides, we possess comprehensive analyzers to conduct properties charactering.

Liposome Homogenizer
This liposome homogenizer has working pressure adjustable between 500 and 30000 psi or 35 and 2000 bar. It has a capacity of 55 L/hr and it can process a sample as small as 250 mL. We have various devices with different processing capabilities, which could meet the different scale of manufacture.

Small-angle X-ray Scattering (SAXS)
SAXS has been used to detect the size and size distribution, also provide information about the unilamellar or multilamellar structure of the liposomes.

Transmission Electron Microscopy (TEM)
This transmission electron microscopy is used for investigation of materials in atomic and micro-scale. TEM can achieve proteins and liposomes visualization which emphasizes the morphology and architecture of liposomes. Normally, cryo-TEM is optimal, but negative staining is an easier and faster procedure. It’s worth noting that the shape of liposome might be distorted during staining and drying process.

Environmental Scanning Electron Microscopy (ESEM)
This equipment has advantages over conventional SEM. It can achieve visualization of liposomes in its natural hydrated state (“nature state”) for liposome formation and morphology. However, the resolution of ESEM analysis do not provide details about the surface properties and architecture of vesicles.

Confocal Microscopy
Structure and architecture of liposomes could be studied in confocal images by labeling the lipidic bilayer with fluorochrome markers. It allows for nondestructive 3D images reconstruction. It is good technique to evaluate the internal structure of large multilamellar vesicles, however, its application is limited by its native resolution which cannot resolve structure sized under 200nm, such as small unilamellar vesicles.

High-performance Liquid Chromatography (HPLC)
This equipment has ability to perform high throughput analysis, which facilitates the calculation of liposome encapsulation efficient (EE). HPLC can be applied for EE determination even if the liposomes do not undergo any purification (e.g. SEC, dialysis). This technique can also be used to monitor the storage stability in terms of leakage or the effect of various disruptive conditions on encapsulated materials.
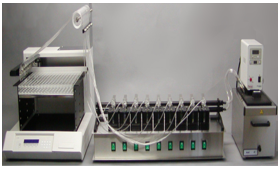
Franz Cell System
This equipment offers an automated solution for drug release test in vitro, provides an accurate and reliable method for evaluating active compound release from liposomes by permeating through the synthetic membrane.
Service
Based on the advanced instruments in our liposome platform, we offer state-of-the-art service that includes but not limited to:
- Design, optimize, and produce liposomes.
- Parameters characterization including size distribution, stability measurement, encapsulation efficiency testing, and so on.
- Measurement of drug release kinetics.
- Customize manufacture is available from mL level to scale up.
Creative Biostructure will support your project with our superb expertise, advanced equipment, and satisfactory after-sales service. We are dedicated to provide optimized and suitable plan to your research program. It is our pleasure to become your trusted partner. Please feel free to contact us for a detailed quote!
Ordering Process
References
- Yan Shen, Yuanxin Ji, Shengjie Xu, et al. (2011) Multivesicular liposome formulations for the sustained delivery of ropivacaine hydrochloride: Preparation, characterization, and pharmacokinetics. Drug Delivery. 18: 361–366.
- Jian QIU, Xiao-hui WEI, Fang GENG, et al. (2005) Multivesicular liposome formulations for the sustained delivery of interferon α-2b. Acta Pharmacologica Sinica.26: 1395–1401.
- Zahra Hadian. (2016) A Review of nanoliposomal delivery system for stabilization of bioactive omega-3 fatty acids. Electronic Physician. 8: 1776-1785.
- Somayeh Hallaj-Nezhadi, Maryam Hassan. (2013) Nanoliposome-based antibacterial drug delivery. Drug Delivery. 2: 1521-0464.

