Exosome Model Evaluation Service
Functional evaluation of cells and animal models in exosome technology services is a key research method. It uses specific cell lines or experimental animals to comprehensively evaluate the various biological functions and potential pharmacological properties of exosomes in vivo and in vitro. This process can not only reveal the nature of exosomes as signal transduction mediators, but also has important significance for exploring their application potential as diagnostic markers or therapeutic carriers. Creative Biostructure's exosome model evaluation service can provide a more comprehensive understanding of the multifaceted potential of exosomes and provide customers with more comprehensive exosome evaluation results.
Functional Evaluation Services for Cell and Animal Models
The technical service project of functional evaluation of cell and animal models is a key step in the comprehensive study of the biological characteristics and potential application value of exosomes. This series of services usually includes the following core links:
- Use efficient and specific methods to extract and purify exosomes from donor cells to ensure the purity and activity of samples.
- Establish appropriate cell lines or construct animal models under specific disease conditions to test the effects of exosomes in an environment close to physiological conditions.
- By introducing exosomes into these model systems, use a variety of experimental techniques such as fluorescent labeling and flow cytometry to observe the interaction process between exosomes and target cells or organisms.
- Based on the above experimental results, conduct in-depth functional and pharmacological property analysis, such as evaluating the effects of exosomes on cell proliferation and apoptosis, or their distribution in the body, targeting and long-term effects.
The entire evaluation process covers a wide range of fields from basic scientific research to preclinical development, which not only helps to reveal the specific mechanism of action of exosomes in different disease contexts, but also provides solid data support for subsequent exploration of exosomes as new diagnostic markers or therapeutic tools.
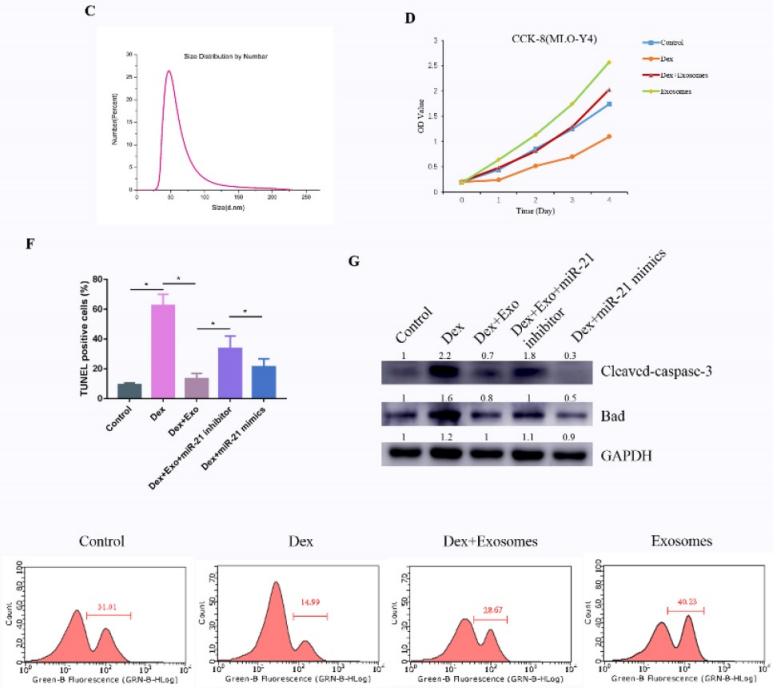 Figure 1. TUNEL assay to detect cell apoptosis. (Kuang M, et al., 2019)
Figure 1. TUNEL assay to detect cell apoptosis. (Kuang M, et al., 2019)
Our Exosome Model Functional Evaluation Services
Creative Biostructure has a complete technology platform and rich service experience, providing a variety of exosome model functional evaluation services.
| Service Content | Description |
| Cell proliferation detection | CCK8 or EDU method was used to evaluate cell proliferation. |
| Cell cycle detection | PI single staining flow cytometry was used to determine the cell cycle stage. |
| Cell apoptosis detection | AnnexinV/PI double staining flow cytometry or TUNEL method was used to determine the cell apoptosis rate. |
| Cell invasion detection | Transwell (matrigel) technology was used to evaluate cell invasion ability. |
| Cell scratch test | Cell scratch test was performed by a tip to measure cell migration performance. |
| Cell inflammatory response | Drug-induced inflammation model was used to detect the pro-inflammatory or anti-inflammatory effects of components such as exosomes. |
| Zebrafish model evaluation | The zebrafish model was used to evaluate exosome whitening, repair, anti-inflammatory and toxicity indicators. |
| Mouse model evaluation | The whitening, repair, anti-inflammatory and toxicity functions of exosomes were investigated using a mouse model. |
Why Choose Creative Biostructure?
- Experienced Research Team: Our team of cell biology, pharmacology, and bioengineering scientists brings decades of experience to every phase of your project evaluation.
- Accurate and Reliable Data: We ensure data accuracy and repeatability through rigorous quality control and verification, ensuring you can rely on our results.
- Cost-Effective and Efficient Services: We are dedicated to delivering fast, cost-effective solutions without compromising quality. Our streamlined workflows and advanced technology reduce turnaround times, providing clients with timely results that align with their research budgets.
- Customized Evaluation Models: Recognizing the unique needs of each project, we offer fully tailored model evaluation solutions. Clients can opt for a range of assays and model types to ensure that their specific research questions are addressed accurately.
Frequently Asked Questions
-
What do I need to provide?
Customers are required to provide exosomes or animal and plant cell samples.
-
What are the results of the delivery?
The delivery results of different testing experiments are slightly different, you can consult us for details.
-
Why choose animal models for exosome evaluation?
Animal models provide an in vivo environment that mimics human physiological conditions, offering insights into exosome distribution, targeting, and long-term therapeutic effects that cannot be observed solely in vitro.
-
What types of cells and animals are used in the models?
We utilize a range of cell lines, including tumor cell lines and primary cells, as well as rodent models like mice and zebrafish. Model selection is based on the research objectives and disease focus to ensure accuracy and relevance.
Creative Biostructure offers high-quality exosome-related services based on professional technical expertise to meet the customized needs of our customers. If you are interested in our services, please contact us for a detailed quote.
Ordering Process
References
- Kuang M, Huang Y, Zhao X G, et al. Exosomes derived from Wharton's jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. International Journal of Biological Sciences. 2019. 15(9): 1861.
- Wu X, Ma Z, Wu D. Derivation of clinical-grade mesenchymal stromal cells from umbilical cord under chemically defined culture condition–platform for future clinical application. Cytotherapy. 2020. 22(7): 377-387.
- Conte M S, Bradbury A W, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. European Journal of Vascular and Endovascular Surgery. 2019. 58(1): S1-S109. e33.
