Introduction: The Foundation of Successful Protein Crystallography
Protein crystallography plays a crucial role in structural biology and drug discovery by revealing the atomic structure of biomolecules. However, obtaining high-quality crystals starts long before crystallization—it begins with optimized protein production and purification. Poor sample quality often leads to heterogeneous proteins, aggregation, or failed crystallization trials, emphasizing the importance of well-prepared proteins.
Key Takeaways for Optimized Protein Preparation
| Factor | Impact on Crystallization | Optimization Strategies |
|---|---|---|
| Protein Construct | Influences solubility and expression | Truncation, mutations, fusion tags |
| Expression System | Affects yield and post-translational modifications | Choose system based on protein type |
| Purification Strategy | Determines purity and homogeneity | Multi-step purification, buffer optimization |
| Stability & Solubility | Essential for successful crystal formation | Chaperones, additives, fusion proteins |
By carefully optimizing these steps, researchers can enhance crystallization success rates and obtain high-resolution structures.
Designing the Optimal Protein Construct
The design of a protein construct is one of the most critical steps in achieving successful protein crystallization. A well-optimized construct can enhance expression, improve solubility, and increase stability, leading to better crystal formation. Conversely, poorly designed constructs can result in aggregation, degradation, or misfolding, ultimately hindering crystallization trials.
 Figure 1. Engineering a Stabilized Minimal Active Construct of Human DDX21. (Chen Z, et al., 2022)
Figure 1. Engineering a Stabilized Minimal Active Construct of Human DDX21. (Chen Z, et al., 2022)
Key Considerations for Construct Design
A rationally designed construct should take into account several factors:
1. Truncation: Removing Flexible or Disordered Regions
Disordered regions, long loops, and flexible termini often interfere with crystallization by introducing heterogeneity and structural instability.
Solution: Use truncation strategies to remove non-essential regions while retaining the functional core of the protein.
- Bioinformatics tools, such as DISOPRED and IUPred, can help predict disordered regions.
- Experimental techniques, such as limited proteolysis followed by mass spectrometry, can identify stable fragments for crystallization.
Case Study: A kinase domain with a disordered N-terminal region failed to crystallize, but after truncating the first 50 residues, high-quality crystals were obtained.
2. Mutations: Engineering Stabilizing Mutations
Certain mutations can enhance protein stability and promote crystallization without disrupting function.
Solution: Introduce rational mutations that:
- Reduce conformational flexibility, such as surface entropy reduction (SER) mutations.
- Enhance thermostability, such as replacing glycine or serine residues in loop regions with proline.
- Stabilize interfaces, especially in oligomeric proteins, by introducing cysteine mutations to promote disulfide bonding.
Example: In G-protein coupled receptors (GPCRs), introducing thermostabilizing mutations allowed researchers to crystallize receptors that previously failed to form ordered crystals.
For innovative strategies to improve protein crystallization success, explore Crystallization with Mutant Library Approaches at Creative Biostructure.
3. Fusion Tags: Improving Solubility and Purification
Fusion tags can enhance expression levels and solubility, preventing aggregation. However, they should be used judiciously as they might interfere with crystallization.
Commonly Used Fusion Tags:
- His-tag: Simplifies affinity purification but should be removed before crystallization.
- Maltose-binding protein (MBP): Improves solubility for difficult proteins. Learn more about our Fusion Protein-Assisted Crystallization service.
- SUMO & GB1: Can stabilize small proteins.
Best Practice: If using a fusion tag, design constructs with cleavable linkers (e.g., TEV or PreScission protease sites) so the tag can be removed before crystallization trials.
4. Optimizing Domains and Cofactors
Multi-domain proteins may require construct engineering to isolate stable domains for crystallization.
Cofactors and ligands often help stabilize proteins in a crystallizable conformation.
Example: The crystallization of kinases and ATP-binding proteins often requires the addition of ATP analogs or inhibitors to lock the protein in a stable state.
For enhanced protein crystallization, explore Co-crystallization and Crystallization Chaperone Strategies.
5. Codon Optimization for Efficient Expression
Even with an ideal construct, poor expression can hinder crystallization. Codon optimization ensures efficient translation, particularly in heterologous expression systems like E. coli or insect cells. Use codon optimization tools to match the expression system's preferred codon usage, ensuring efficient translation and higher protein yields. Creative Biostructure offers Custom Codon Optimization Services to tailor gene sequences for optimal expression, and our Codon Usage Frequency Table provides valuable insights into species-specific codon preferences.
By implementing truncation strategies, stabilizing mutations, solubility-enhancing fusion tags, and codon optimization, researchers can significantly improve protein yield, homogeneity, and crystallization success rates.
Selecting the Right Expression System
Choosing the appropriate protein expression system is a crucial step in obtaining high-quality samples for crystallization. The expression system determines yield, solubility, post-translational modifications (PTMs), and overall structural integrity, all of which influence crystallization success. Different proteins have varying requirements, making it essential to evaluate the advantages and limitations of each system before production begins.
Key Factors in Selecting an Expression System
When deciding on an expression system for protein crystallization, researchers should consider:
- Protein complexity and size: Simple bacterial systems work well for small, soluble proteins, while eukaryotic systems are better for complex, multi-domain proteins.
- Post-translational modifications (PTMs): If glycosylation, phosphorylation, or disulfide bonding is required, a mammalian or insect system may be necessary.
- Solubility and folding: Some proteins require chaperones or specific folding conditions, which influence the choice of system.
- Yield and scalability: High-throughput crystallography projects often demand large amounts of purified protein.
Comparison of Protein Expression Systems
| Expression System | Advantages | Limitations |
|---|---|---|
| Bacterial (E. coli) | High yield, fast growth, low cost, simple genetic manipulation | Lacks PTMs, solubility issues for large or complex proteins, potential inclusion body formation |
| Yeast (Pichia pastoris, S. cerevisiae) | Eukaryotic-like PTMs, good scalability, secretes proteins into media | Glycosylation patterns may differ from higher eukaryotes, lower yield than bacteria |
| Insect (Baculovirus-infected Sf9/Sf21, Hi5) | Proper folding and PTMs, high protein quality, good expression of membrane proteins | Requires virus handling, longer production time, costlier than bacteria |
| Mammalian (HEK293, CHO cells) | Native PTMs, best for complex and therapeutic proteins, high functional activity | Expensive, lower yield, difficult scalability |
Explore Comparison of Protein Expression Systems to find the best option for your crystallography needs.
Protein Purification: Strategies for High-Quality Samples
Protein purification is a critical step in structural biology, as the quality of the purified protein directly impacts the success of crystallization and subsequent structure determination. Achieving high purity, homogeneity, and stability is essential for generating well-diffracting crystals. This section explores key purification techniques, assessment methods, and optimization strategies to obtain crystallization-grade protein samples.
The Importance of Protein Purification for Crystallography
Protein crystallization is highly sensitive to contaminants, heterogeneity, and aggregation, making purification a crucial process. The goals of protein purification for crystallography include:
- High purity: Removing unwanted contaminants ensures reproducibility in crystallization trials.
- Monodispersity: Uniform protein samples improve crystal formation and diffraction quality.
- Stability: Maintaining protein integrity throughout purification prevents degradation or aggregation.
- Sufficient yield: Adequate protein amounts are required for screening multiple crystallization conditions.
To achieve these objectives, a combination of chromatographic techniques and rigorous quality control assessments must be employed.
Common Protein Purification Strategies
Protein purification typically follows a multi-step strategy to isolate the target protein while minimizing contaminants. The process often includes:
1. Affinity Chromatography: High-Specificity Capture
Affinity chromatography is the most commonly used first-step purification technique due to its high specificity and efficiency.
| Advantages |
|
| Limitations |
|
| Common Affinity Tags |
|
| Optimization Tips |
|
2. Ion Exchange Chromatography (IEX): Charge-Based Separation
IEX separates proteins based on their net charge at a given pH, allowing the removal of contaminants and achieving high purity.
| Advantages |
|
| Limitations |
|
| Types of IEX |
|
| Optimization Tips |
|
3. Size Exclusion Chromatography (SEC): Purification by Molecular Size
SEC (also called gel filtration chromatography) separates proteins based on their hydrodynamic radius, allowing for final polishing and removal of aggregates.
| Advantages |
|
| Limitations |
|
| Optimization Tips |
|
Quality Control: Assessing Protein Purity and Homogeneity
Purified protein must be rigorously assessed to ensure its suitability for crystallization. Key analytical techniques include:
SDS-PAGE & Western Blot: Confirms protein purity and verifies molecular weight.
Mass Spectrometry (MS): Confirms correct protein identity and detects modifications.
Dynamic Light Scattering (DLS): Detects aggregation and measures monodispersity.
Circular Dichroism (CD) Spectroscopy: Evaluates protein secondary structure and folding integrity.
Thermal Shift Assay (DSF): Assesses protein stability under varying buffer conditions.
For crystallization, DLS and SEC profiles must indicate monodisperse samples with minimal aggregation.
Overcoming Common Challenges in Protein Purification
Even with optimized protocols, challenges may arise during purification. Here are some common problems and solutions:
| Issue | Cause | Solution |
|---|---|---|
| Protein degradation | Proteolysis during purification | Add protease inhibitors (PMSF, EDTA), perform purification at 4°C |
| Low yield | Poor expression or loss during purification | Optimize expression conditions, use gentle elution strategies |
| Aggregation | High protein concentration, improper buffer | Use SEC for monodispersity, optimize buffer with additives (e.g., glycerol, arginine) |
| Poor binding in affinity chromatography | Improper tag exposure or weak binding | Test different resin types, optimize pH and salt concentration |
| Precipitation after tag removal | Protein stability issue | Adjust pH, salt concentration, or try alternate tag-free expression |
Successful protein purification for crystallography requires a combination of affinity purification, charge-based separation, and size exclusion chromatography, followed by thorough quality control assessments. By optimizing purification protocols and buffer conditions, researchers can obtain highly pure, stable, and monodisperse protein samples ready for crystallization trials.
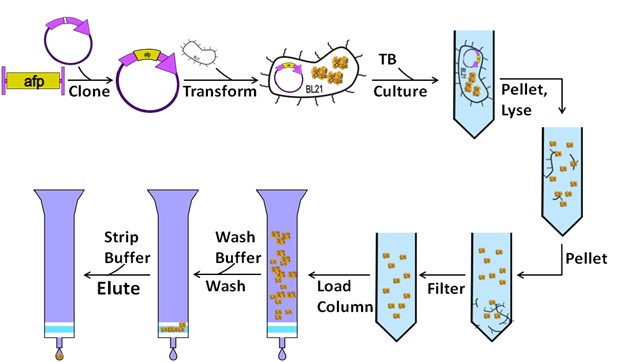
 Figure 2. Schematic representation of the DDX21 expression and purification process, including cell preparation, affinity purification, and size exclusion chromatography. (Chen Z, et al., 2022)
Figure 2. Schematic representation of the DDX21 expression and purification process, including cell preparation, affinity purification, and size exclusion chromatography. (Chen Z, et al., 2022)
Overcoming Solubility and Stability Challenges
Achieving high solubility and stability is one of the biggest challenges in protein production and purification for crystallography. Many proteins tend to aggregate, degrade, or lose their functional conformation, making crystallization difficult. This section explores the key challenges, proven strategies, and practical solutions to enhance protein solubility and stability for successful crystallization.
Why Are Solubility and Stability Crucial for Crystallography?
Protein solubility and stability directly affect crystallization success. Poorly soluble or unstable proteins can lead to:
Aggregation: Prevents uniform crystal formation.
Precipitation: Leads to protein loss and inconsistent crystallization results.
Conformational changes: Impairs diffraction quality in X-ray crystallography.
Degradation: Produces heterogeneous protein populations, reducing crystallization efficiency.
To overcome these challenges, various strategies can be applied during expression, purification, and storage to ensure that the protein remains soluble, stable, and crystallization-ready.
Strategies to Improve Protein Solubility
Solubility issues often arise from protein aggregation, incorrect folding, or exposure of hydrophobic regions. Below are key methods to enhance solubility:
1. Optimizing Expression Conditions
Lower induction temperature (e.g., 16°C instead of 37°C in bacterial expression systems) allows slower folding, reducing aggregation.
Use co-expression with molecular chaperones (e.g., GroEL/GroES, DnaK, Hsp70) to assist proper folding.
Modify culture media composition (e.g., addition of osmolytes, amino acids) to enhance solubility.
2. Using Solubility-Enhancing Tags
Fusion tags can improve protein folding and solubility:
MBP (Maltose-Binding Protein): Strongly enhances solubility.
GST (Glutathione S-Transferase): Helps stabilize aggregation-prone proteins.
SUMO (Small Ubiquitin-like Modifier): Enhances proper folding and stability.
Trx (Thioredoxin): Prevents misfolding in disulfide bond-containing proteins.
Tags can be removed enzymatically (e.g., TEV, thrombin, Factor Xa cleavage) before crystallization.
3. Buffer Optimization for Solubility
Salt concentration: Moderate levels (100-300 mM NaCl or KCl) prevent aggregation.
Detergents: For membrane proteins, detergents like DDM, CHAPS, or OG stabilize hydrophobic regions.
Osmolytes and additives: Compounds like glycerol (5-20%), arginine (50-500 mM), or proline enhance solubility.
pH tuning: Adjust pH close to protein's pI (isoelectric point) to minimize aggregation risks.
4. Refolding Strategies for Inclusion Bodies
Some proteins form inclusion bodies (insoluble aggregates) in bacterial expression systems.
Denaturation-renaturation approaches using chaotropic agents (urea, guanidine-HCl) followed by stepwise dialysis can improve solubility.
Adding redox pairs (DTT/GSH) can assist in disulfide bond formation.
Strategies to Improve Protein Stability
Even if a protein is soluble, instability can lead to degradation or loss of function. Stability improvements involve buffer formulation, storage conditions, and structural modifications.
1. Buffer Optimization for Stability
pH Stability: Choose a pH range that minimizes denaturation (typically pH 6.5-8.5 for most proteins).
Reducing agents: Add DTT (1-5 mM), TCEP (0.5-2 mM), or β-mercaptoethanol to maintain disulfide bond integrity.
Glycerol (5-20%): Protects against freeze-thaw damage.
Sugars (trehalose, sucrose, sorbitol): Prevents aggregation and stabilizes protein structure.
Metal ions: Some proteins require cofactors (e.g., Mg²⁺, Zn²⁺) to maintain native structure.
2. Preventing Proteolysis and Degradation
Include protease inhibitors (e.g., PMSF, EDTA, AEBSF) in purification buffers.
Use low-temperature purification (4°C) to slow down proteolytic activity.
Shorten purification time to reduce exposure to proteases.
Modify protein sequence by removing unstable or flexible regions (e.g., disordered N-/C-terminal tails).
3. Storage and Long-Term Stability
Flash-freezing in liquid nitrogen (-80°C) prevents protein degradation.
Use lyophilization (freeze-drying) for long-term stability, if compatible.
Store proteins in small aliquots to avoid repeated freeze-thaw cycles.
4. Experimental Methods to Assess Solubility and Stability
To ensure that a purified protein is suitable for crystallization, several analytical techniques can be used to assess solubility, stability, and homogeneity:
Dynamic Light Scattering (DLS): Detects aggregation and measures polydispersity.
Size-Exclusion Chromatography (SEC): Evaluates monodispersity and oligomeric state.
Thermal Shift Assay (DSF): Determines protein stability by measuring unfolding temperatures.
Circular Dichroism (CD) Spectroscopy: Assesses secondary structure integrity.
Limited Proteolysis: Tests protein stability by exposing to proteases and analyzing degradation patterns.
These techniques help identify optimal buffer conditions, storage conditions, and stabilizing agents for maximizing protein quality.
Overcoming solubility and stability challenges is essential for obtaining high-quality protein samples suitable for crystallography. By fine-tuning expression conditions, selecting appropriate purification strategies, and optimizing buffers, researchers can significantly improve protein behavior and crystallization success.
Scaling Up: From Small-Scale to Large-Scale Production
Scaling up protein production is a crucial step for structural biology studies. While small-scale expression and purification are sufficient for initial screenings, large-scale production is required for obtaining the highly concentrated and pure protein samples needed for crystallization trials. This section explores the key considerations, strategies, and tools to successfully transition from small-scale to large-scale protein production.
Key Considerations for Scaling Up Protein Production
When scaling up protein production, maintaining consistency, yield, purity, and functionality is essential. Several factors need to be optimized, including:
- Expression System Suitability: Some systems scale up better than others (e.g., bacterial vs. mammalian).
- Bioreactor vs. Flask Culture: Scaling up requires choosing between larger flask cultures or bioreactor-based systems.
- Growth Conditions: pH, temperature, aeration, and media composition must be optimized.
- Downstream Processing: Larger volumes require efficient purification methods to maintain yield and quality.
- Cost Efficiency: Resources, reagents, and time should be managed effectively.
Each step of the process must be optimized and validated to ensure that the scaled-up production maintains the same quality and activity as small-scale preparations.
Optimizing Expression Systems for Large-Scale Production
Different expression systems exhibit varying scalability. Choosing the most appropriate system depends on factors like protein complexity, post-translational modifications, and yield requirements.
For large-scale production, bacterial and yeast systems are generally preferred due to their ease of scaling, low cost, and fast growth. However, insect and mammalian cells are essential for producing proteins requiring proper folding, glycosylation, or membrane integration.
Strategies for Efficient Scale-Up in Bioreactors
When moving from small-scale (e.g., shake flasks) to large-scale (e.g., 5 L-1000 L bioreactors), optimizing growth conditions, aeration, and feeding strategies is key to maximizing protein yield and quality.
1. Choosing the Right Bioreactor System
There are several bioreactor types to consider for large-scale protein production:
Stirred-tank bioreactors: Most commonly used, offers controlled pH, oxygen, and temperature.
Wave bioreactors: Suitable for suspension mammalian/insect cell cultures, reducing shear stress.
Perfusion bioreactors: Maintain high cell density for continuous production.
For bacterial and yeast expression, stirred-tank bioreactors are typically preferred.
For insect and mammalian cells, wave and perfusion bioreactors provide better viability and productivity.
2. Optimizing Growth Conditions for Scale-Up
Oxygen supply: Larger cultures require higher oxygen transfer rates (OTR) for efficient cell growth.
pH and temperature control: Fine-tuning pH (e.g., pH 6.8–7.2 for mammalian cells) and temperature ensures optimal expression.
Induction timing: Large-scale IPTG or methanol induction must be precisely controlled to prevent overburdening cells.
3. Fed-Batch vs. Continuous Culture
| Culture Type | Description | Pros | Cons |
|---|---|---|---|
| Batch Culture | Cells grow in a fixed volume of media until nutrients deplete. | Simple, easy to set up. | Limited yield, inconsistent growth. |
| Fed-Batch Culture | Nutrients are added gradually, extending growth phase. | Higher cell density, increased protein yield. | Requires careful control of nutrient addition. |
| Continuous Culture (Perfusion) | Cells are constantly fed while waste is removed. | Sustained high yield, minimal waste. | More complex, requires automated systems. |
For large-scale production, fed-batch culture is most commonly used due to its ability to increase protein yield while remaining cost-effective.
4. Scaling Up Purification: High-Throughput Approaches
Purification at larger scales requires high-throughput, efficient, and reproducible methods.
(1) Automated Protein Purification Systems
Fast Protein Liquid Chromatography (FPLC): Essential for large-scale affinity, ion-exchange, and size-exclusion chromatography.
Robotic purification platforms: Allow for parallel processing of multiple samples, increasing throughput.
Using FPLC and automated systems ensures that purity and yield are maintained, even at high volumes.
(2) Cost-Effective Purification Strategies
To manage cost while ensuring purity:
Use high-capacity resins (e.g., Ni-NTA for His-tagged proteins) to reduce resin consumption.
Optimize buffer compositions to minimize waste and increase resin reusability.
Implement scalable dialysis or ultrafiltration for buffer exchange instead of expensive desalting columns. Choosing cost-effective purification methods reduces expenses while maintaining protein quality.
Future Trends in Protein Production and Purification
The future of protein production and purification for crystallography is being shaped by advancements in automation, artificial intelligence, and synthetic biology. High-throughput robotic systems are streamlining protein expression and purification workflows, reducing manual effort and increasing reproducibility. AI-driven optimization tools are improving construct design, buffer selection, and purification strategies, leading to higher yields and better sample quality. Additionally, cell-free expression systems and engineered host strains are expanding the possibilities for producing complex or previously intractable proteins. As these technologies continue to evolve, they promise to enhance efficiency, scalability, and success rates in structural biology research.
Conclusion: Optimizing for Crystallography Success
Producing high-quality, crystallizable proteins requires careful construct design, expression system selection, purification optimization, and solubility enhancement. By implementing these strategies, researchers can maximize crystallization success and obtain high-resolution structures. Need expert support? Creative Biostructure offers comprehensive protein production and protein crystallization services to accelerate your research. Contact us today and take your structural biology projects to the next level.
References
- Schwarzenbach D. The success story of crystallography. Foundations of Crystallography. 2012, 68(1): 57-67. https://doi.org/10.1107/S0108767311030303
- Dauter Z, Wlodawer A. Progress in protein crystallography. Protein and peptide letters. 2016, 23(3): 201-210.
- Chen Z, Huang J, Li J. Protein purification, crystallization, and structure determination of human DEAD-box RNA helicase DDX21 in different unwinding states. STAR protocols. 2022, 3(3): 101642. https://doi.org/10.1016/j.xpro.2022.101642
- Timofeev V, Samygina V. Protein crystallography: achievements and challenges. Crystals, 2023, 13(1): 71. https://doi.org/10.3390/cryst13010071