CPMG Relaxation Dispersion NMR Service
Protein oligomerization in cells is important for both health and disease, so it is crucial to understand the mechanisms by which proteins can bind themselves. The initial stages of the oligomerization process are difficult to detect because such states exist for only a fraction of a second. It usually involves the formation of sparse and transient states that are difficult to be characterized by standard biophysical methods but may have a critical biological role.
Creative Biostructure offers CPMG relaxation dispersion NMR services that enable high-resolution structural determination of proteins in low-abundance, transient, high-energy states. We provide unique atomic resolution information on the structure, dynamics, and thermodynamics of protein conformational equilibria occurring on the µs-ms time scale. We provide our customers with insight into the impact of small changes in protein shape on its function.
Applications
- Determine the PCS of a protein in its high energy state
- Provide a comprehensive characterization of conformational equilibria, returning structural, kinetic, and thermodynamic information about the exchange process
- Characterize conformational equilibria occurring on the μs-ms time scale
General Process
- Protein sample preparation
- Step-by-step experimental parameter setting
- Data analysis
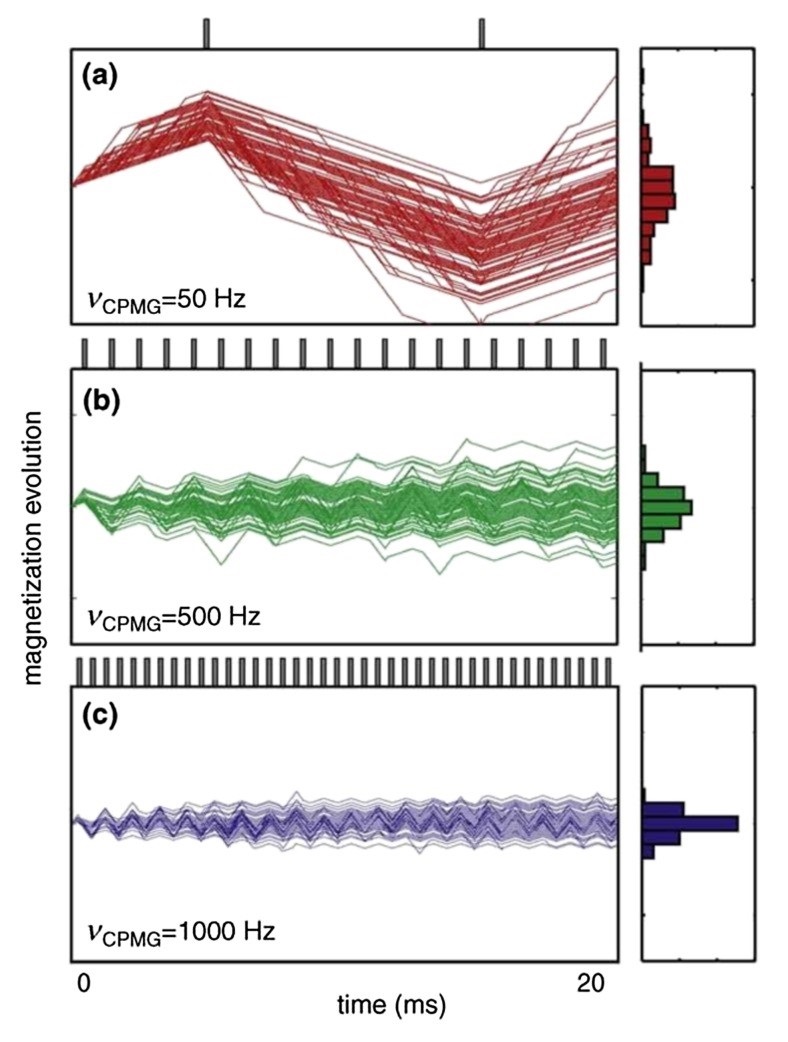 Figure 1. CPMG relaxation dispersion experiments. (Farber, P. J.; et al. 2015)
Figure 1. CPMG relaxation dispersion experiments. (Farber, P. J.; et al. 2015)
Technical Advantages
- Detect a large number of residues individually in a single experiment
- Have versatility for different protein types and is also applicable to smaller proteins
- Detect low levels (0.5% or higher) of intermediates and other excited states that are not directly observable in the spectrum
- Determine small structural changes very accurately and can capture the structure of high energy states present on microsecond to millisecond time scales
- Relatively easy to implement on modern NMR spectrometers and does not require specialized sample preparation steps (i.e., crystallization, sample freezing or alignment, and/or covalent binding to fluorescent or paramagnetic tags)
- Multi-media compatible, including proteins in solution
Creative Biostructure offers the CPMG relaxation dispersion NMR service to determine the corresponding protein high-energy structures. Detailed atomic resolution information on the millisecond time scale dynamics behind the metastability can be provided. This will greatly increase our customers' understanding of protein function at atomic resolution and open the door to the rational design of complex protein functions. Feel free to contact us to address any of your related needs.
Ordering Process
Reference
- Farber, P. J.; et al. Relaxation dispersion NMR spectroscopy for the study of protein allostery. Biophysical Reviews. 2015, 7(2): 191-200.
