Non-PEGylated Liposomes (NPL) Production
Possessing several liposome technology platforms, Creative Biostructure offers professional liposome manufacture service for your research or drug development. Based on deep understanding of the liposomal preparation and application, we customize Non-PEGylated liposomes(NPL) delivery system to overcome the defects of PEGylated liposomes using the targeted technology.
How Non-PEGylated Liposomes Work
PEGylated liposomes are generally thought to reduce immunogenicity of the vesicles and prolong the circulation time in vivo, and have been widely recognized as one of the most successful strategies to improve the delivery of therapeutic molecules. However, PEGylated-liposomes have recently been demonstrated to show side effects. For example, PEGylated liposomal doxorubicin presents palmar plantar erythrodysesthesia while the Non-PEGylated formula does not. Our non-PEGylated liposome (NPL) technology has been established to provide an alternative carrier with lower toxicity allowing for a higher accumulative dose.
Non-PEGylated Liposomes Applications
In order to produce high-quality Non-PEGylated liposomes, Creative Biostructure uses quality phospholipids from natural and synthetic sources. Creative Biostructure also provides customized NPL with specific characteristics such as various liposome sizes, drug to lipid ratio, lipid saturation level. The lipid composition could be further designed to include reactive lipids on the outer surface that allows conjugation of different molecules such as antibodies, peptides, or proteins. Besides, our extensive manufacturing processes support different scales of production for further development and commercialization.
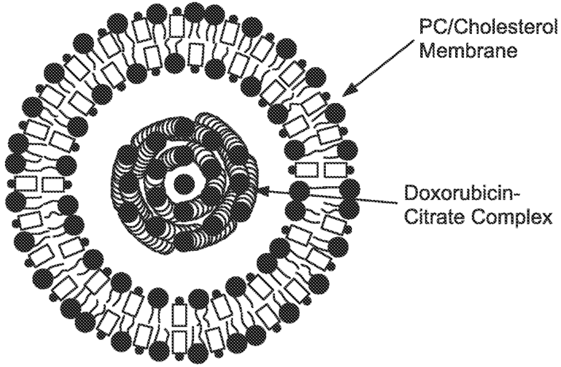 Figure 1. Non-PEGylated liposome doxorubicin. (US20110256215 A1, 2011)
Figure 1. Non-PEGylated liposome doxorubicin. (US20110256215 A1, 2011)
Strategies for Non-PEGylation Liposome Manufacture
- Thin film evaporation
- High-pressure homogenizer
- Reverse phase evaporation vesicles
- Ethanol injection-extrude & modified ethanol injection-extrude
- Microemulsion cooling
- Freeze-thawing & lyophilization
Precise analyses are also available at Creative Biostructure. Our advantageous tests are listed below:
- Appearance morphology test by transmission electron microscopy (TEM) or scanning electron microscopy (SEM)
- Zeta potential to predict the stability of liposomes
- Encapsulation efficiency (ER) & drug loading capacity (LC)
- In vitro drug release & in vivo pharmacokinetics
Creative Biostructure has been well recognized in liposome and other lipid-based products manufacturing. Relying on the first-in-class expertise, our technical team can customize various classifications of liposomes which are suitable for different drug delivery systems. Please do not hesitate to consult with us through online inquiry.
Ordering Process
References
- Amy Y. Grahn, Krystof S. Bankiewicz, Millicent Dugich-Djordjevic, et al. (2009) Non-PEGylated liposomes for convection-enhanced delivery of topotecan and gadodiamide in malignant glioma: initial experience. Journal of Neuro-Oncology. 95:185–197.
- Janina brucker, Christine mayer, Gerhard gebauer, et al. (2016) Non‑pegylated liposomal doxorubicin for patients with recurrent ovarian cancer: A multicentric phase II trial. Oncology letters.12: 1211-1215.
