Lyophilized Microvesicles
Lyophilization (freeze-drying) is a technique which makes microvesicles more stable and easier to store and transport. This process removes water from the vesicles, preserving the integrity of the structure and functional properties at 4 °C for long-term storage.
Research and clinical uses of lyophilized microvesicles are exploding as the bioactive component of very convenient, easy to handle and transport, less degradable and longer-lasting bioactive microvesicles. They are used in diagnostics, drug delivery, and therapy, making them a necessary part of today's biomedical research. Creative Biostructure has several lyophilized exosome standards available for research and offers exosome isolation services to meet your needs.
Product List
Background
Lyophilization (freeze-drying) is applied to stabilize microvesicles. The vacuum extraction process used in lyophilization removes water, leaving the powder dry without losing the structure and function of the vesicles. The procedure considerably extends microvesicles' shelf life and makes them easier to store and transport without refrigeration. For a quick refresher on how exosomes are lyophilized:
Preparation: Exosome sample should be pre-suspended in appropriate lyophilization buffer first. This buffer usually includes cryoprotectants (trehalose or mannitol) to shield the exosomes during the freezing and drying phases because they avoid structural disruption and maintain bioactivity.
Freezing: The sample is immediately frozen (often at very low temperatures (-40 °C to -80 °C) to freeze the exosome solution. Rapid freezing also preserves the structural integrity of the exosomes by forming smaller ice crystals.
Primary Drying (Sublimation): After freezing, the specimen is placed in a lyophilizer with vacuum. When placed at these low pressures, the frozen water in the sample sublimates, leaving the dry exosome particles behind. This step is carefully controlled to avoid damaging the exosomal structure.
Secondary Drying (Desorption): In this step, trapped water is gradually evaporated by heating and vacuuming. This process leaves the final product completely dry for greater stability and long-term storage.
Storage: Lyophilized exosome powder should be stored in a sealed, sterile container. It can be reconstituted with a buffer or medium as needed for downstream applications.
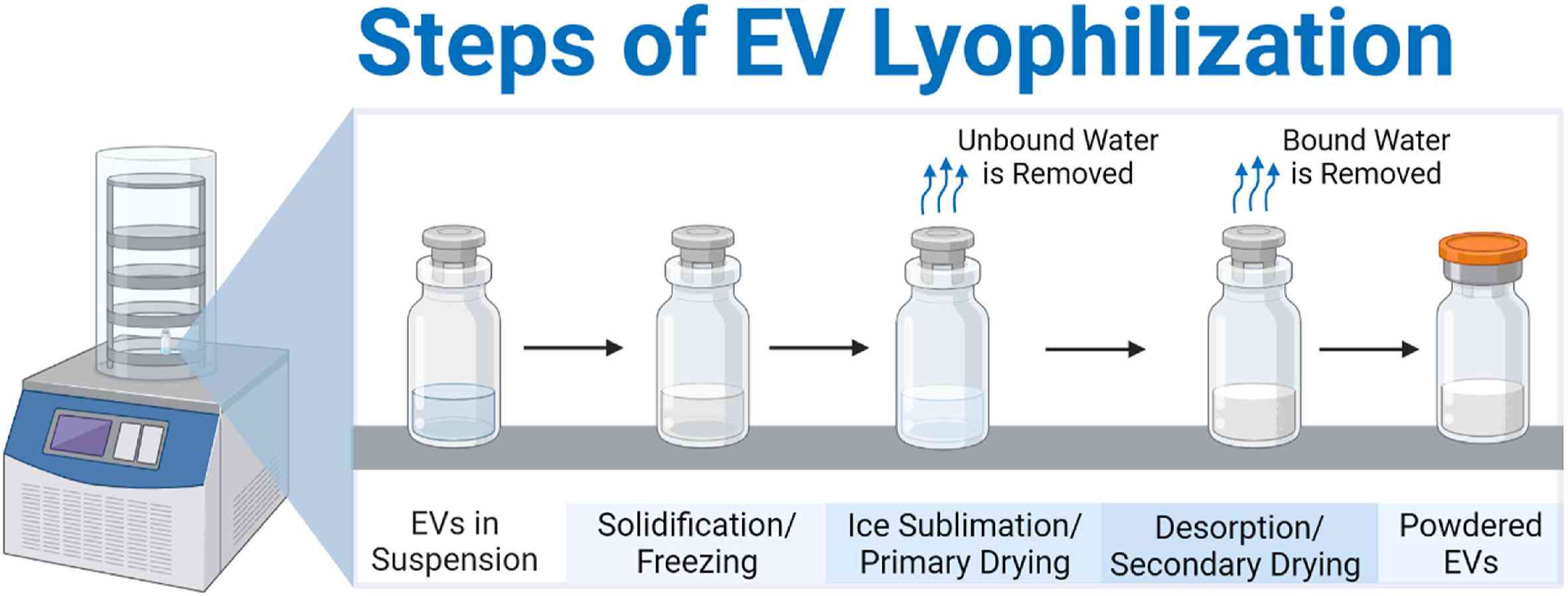 Figure 1. The steps of lyophilization for extracellular vesicle preparations, resulting in a dry, powdered extracellular vesicles (EV) product. (Golan ME and Stice SL, 2024)
Figure 1. The steps of lyophilization for extracellular vesicle preparations, resulting in a dry, powdered extracellular vesicles (EV) product. (Golan ME and Stice SL, 2024)
Applications of Lyophilized Microvesicles
Diagnostics
Lyophilized microvesicles are being used as powerful biomarkers for specific diseases. Some miRNAs have been shown to be useful diagnostic markers for non-invasive monitoring of cancer status. There are also exosomal proteins, such as tetraspanins, which have been shown to be biomarkers of cardiovascular disease and could aid in detection and prognosis.
Drug Delivery
The lyophilized microvesicles can contain multiple therapies, from small molecules to RNA therapy and proteins. For example, we know that microvesicles can deliver siRNA directly to cells to silence genes in liver cancer models. Their lipid bilayer not only protects the encapsulated cargo from enzymatic degradation, but also facilitates cellular transport via receptor-mediated endocytosis. This specificity enables targeted therapy and eliminates the common side effects associated with conventional drug delivery.
Therapeutic Research
Currently, there are ongoing studies of lyophilized microvesicles as regenerative medicine agents. A notable example is the use of mesenchymal stem cell (MSC)-derived microvesicles to promote tissue regeneration. In preclinical research, MSC-derived microvesicles promote cardiac repair after myocardial infarction by delivering growth factors and anti-apoptotic signals to damaged tissue. In addition, such microvesicles have been shown to be immunomodulatory, reducing inflammation and healing old wounds, opening up new therapeutic possibilities.
Case Studies
Case Study 1: Lyophilized Microvesicles from Mesenchymal Stem Cells
Mesenteric stem cell exosomes are increasingly being used as an immunogenic, cell-free tissue engineering therapy that could carry a regenerative cargo. They have been shown in preclinical studies to promote wound healing, bone regeneration and cartilage repair by transferring bioactive proteins, mRNAs and microRNAs. These exosomes regulate inflammation, angiogenesis, cell growth and matrix production—making them a more robust substitute for whole stem cells. They are promising, but not yet ready for commercialization in isolation, mechanistic explanation or human trials. Improved exosome loading, production and biodistribution are key to clinical translation.
In terms of storage, lyophilization extends the shelf life of exosomes by several factors: freeze-dried mesenchymal stem cell (MSC) exosomes are stable and bioactive for six months at -20 °C, refrigerated exosomes for about a week, and frozen exosomes for three months. The freeze-thaw cycle does not degrade exosomes as much as it does live MSCs. This stability suggests that exosome treatments may overcome some of the storage and shelf-life limitations of conventional cell-based products.
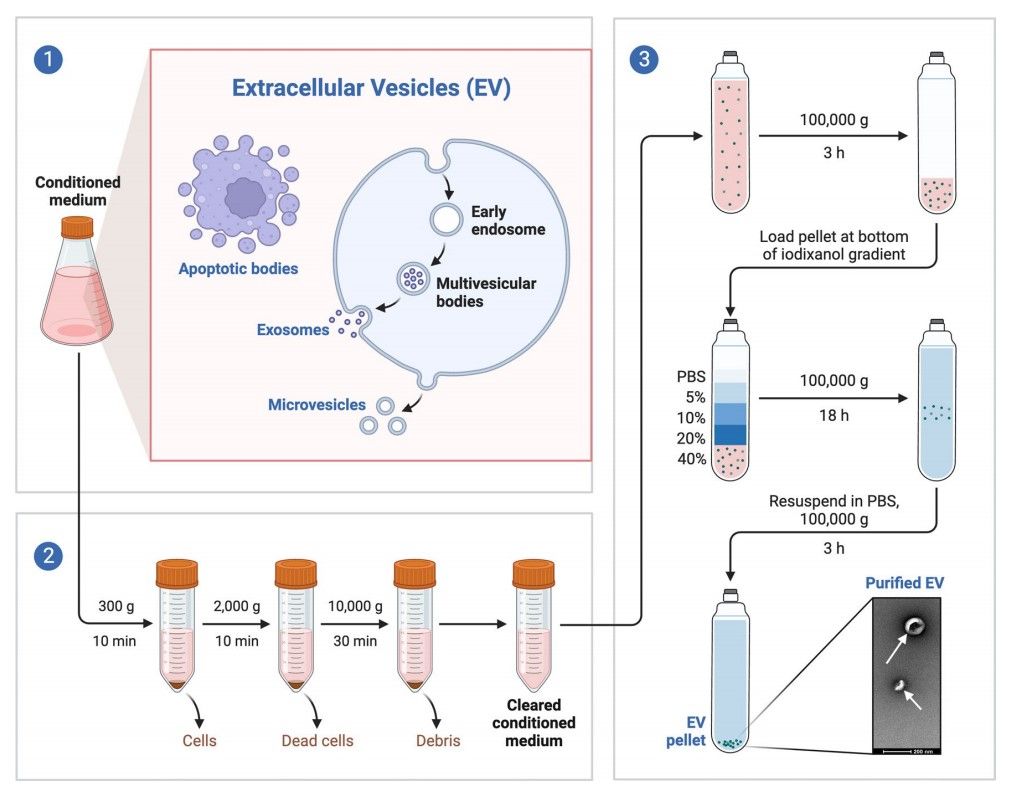 Figure 2. (1) Extracellular Vesicles (EV) are released by cells during their normal activity. (2) For EV separation, conditioned culture medium is harvested and major contaminants removed by consecutive low speed centrifugations. (3) The cleared supernatant is concentrated by ultracentrifugation, and the resulting 100K pellet is loaded on the bottom of an iodixanol gradient. EVs float upwards and EV-enriched fractions are collected and pelleted. The final sample is rich in EVs and absent in contaminants. (Roszkowski S, 2024)
Figure 2. (1) Extracellular Vesicles (EV) are released by cells during their normal activity. (2) For EV separation, conditioned culture medium is harvested and major contaminants removed by consecutive low speed centrifugations. (3) The cleared supernatant is concentrated by ultracentrifugation, and the resulting 100K pellet is loaded on the bottom of an iodixanol gradient. EVs float upwards and EV-enriched fractions are collected and pelleted. The final sample is rich in EVs and absent in contaminants. (Roszkowski S, 2024)
Case Study 2: Study on the Preparation of Bovine Milk Exosomes and the Stability of Lyophilized Powder
MK-exo, or milk-derived exosomes from bovine sources, are active molecules and beneficial to human health. In addition, MK-exos can be used in drug delivery. But their preservation and transport currently present challenges. In this research, high quality MK-exo was produced by large-scale preparation and a freeze-drying protocol for MK-exo was invented. In the absence of accelerated or long-term stability testing for lyophilized MK-exo, the data indicate that lyophilized MK-exo are stable at room temperature for at least 3 months and at 2-8 °C for up to 15 months. This demonstrates that lyophilization provides good stability for MK-exo, which can be easily stored and transported over time.
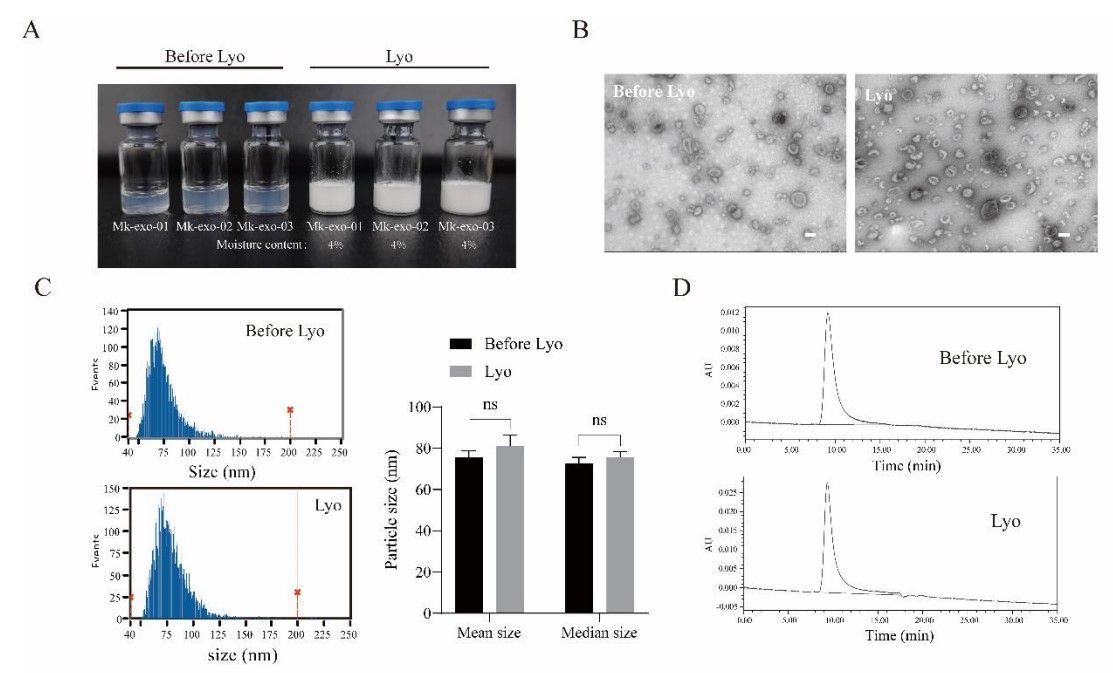 Figure 3. Characterization of liquid and lyophilized MK-exo. (A) The photo of liquid and lyophilized MK-exo; (B-D) The main morphological features, particle size analysis and proteome Wayne diagram of liquid and lyophilized MK-exo, Scale bar = 100 nm. (Lu L, et al., 2023)
Figure 3. Characterization of liquid and lyophilized MK-exo. (A) The photo of liquid and lyophilized MK-exo; (B-D) The main morphological features, particle size analysis and proteome Wayne diagram of liquid and lyophilized MK-exo, Scale bar = 100 nm. (Lu L, et al., 2023)
Product Advantages
- Enhanced Stability: Our lyophilized microvesicles are specially treated to remove water, increasing their stability and significantly reducing the risk of degradation. This guarantees their stability and bioactivity even during long-term storage, making them a good source of quality for the most sensitive research.
- Extended Shelf Life: Our lyophilized powder has been developed with the utmost care for long-term stability and quality, so that it retains its integrity and consistency even after prolonged storage. When stored at 4 °C, the powder will remain fully active and stable for a long time—perfect for laboratories and research institutes where materials need to perform reliably over time.
- Effortless Handling and Reconstitution: Offered in a convenient powder form, our microvesicles are easy to handle and can be readily reconstituted in buffers, streamlining experimental workflows and minimizing prep time.
- Broad Application Range: Our lyophilized microvesicles are suitable as standard controls for a wide range of applications, including diagnostics (as biomarkers), targeted drug delivery, and cellular communication research. Their stability and bioactivity ensure high performance in various cellular and molecular studies, making them a versatile solution for advancing therapeutic research.
Resources
Frequently Asked Questions
-
What are the storage conditions for lyophilized microvesicles?
For longer storage, lyophilized microvesicles should be stored at 4 °C and frozen liquid at -20 °C to -80 °C. Avoid multiple freeze-thaw cycles.
-
What should be the protocol for reconstitution of lyophilized microvesicles?
Reconstitute lyophilized exosomes by adding deionized water to desired final concentration. Centrifuge before opening to ensure exosomes are at the bottom, resuspend exosomes by pipetting and/or vortexing, please avoid bubbles. Centrifuge again and mix well before use.
-
What quality control measures are used to ensure the integrity of lyophilized microvesicles?
Each batch of lyophilized microvesicles produced is subjected to quality control testing to confirm size, integrity, and bioactivity, to ensure consistent performance after lyophilization. Our quality control platform is powered by an extensive system of more than 60 exosome quality assays, already validated and continuously being improved.
Explore our lyophilized microvesicle products and customized services designed for stability and convenience. Contact us to find the ideal solution for your applications!
References
- Golan ME, Stice SL. Extracellular vesicle lyophilization for enhanced distribution to the point of care. Extracellular Vesicle. 2024;3:100041.
- Lu L, Han C, Wang M, et al. Study on the preparation of bovine milk exosomes and the stability of lyophilized powder. bioRxiv. 2023.
- Roszkowski S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin Exp Med. 2024;24(1):46.


