Articles
Talk With An Expert
- All
- AFM
- AI
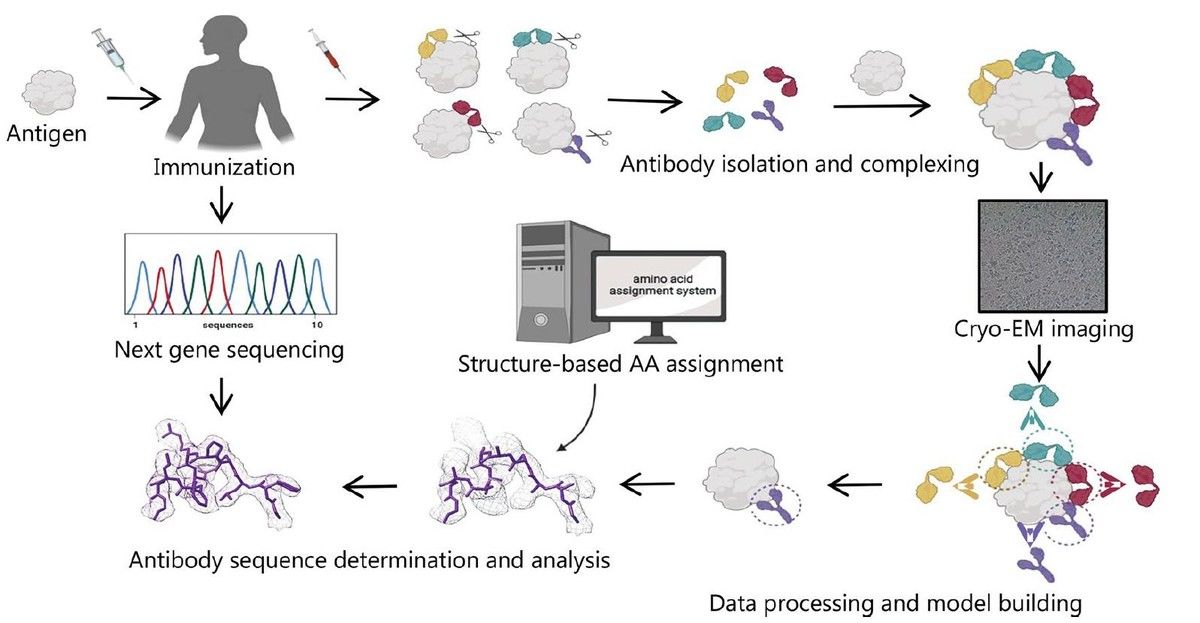
- Antibody
- Bioconjugation
- Carbohydrate
- Cryo-EM
- Cryo-ET
- Crystallography
- Data Processing
- DNA
- Drug Development
- FTIR
- ICP-MS
- Microarray
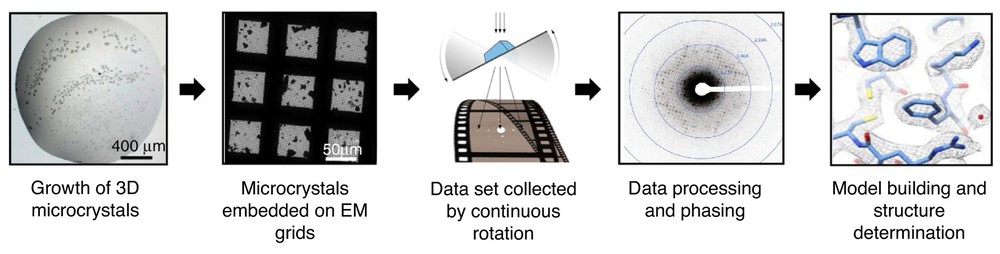
- MicroED
- Molecular Dynamics
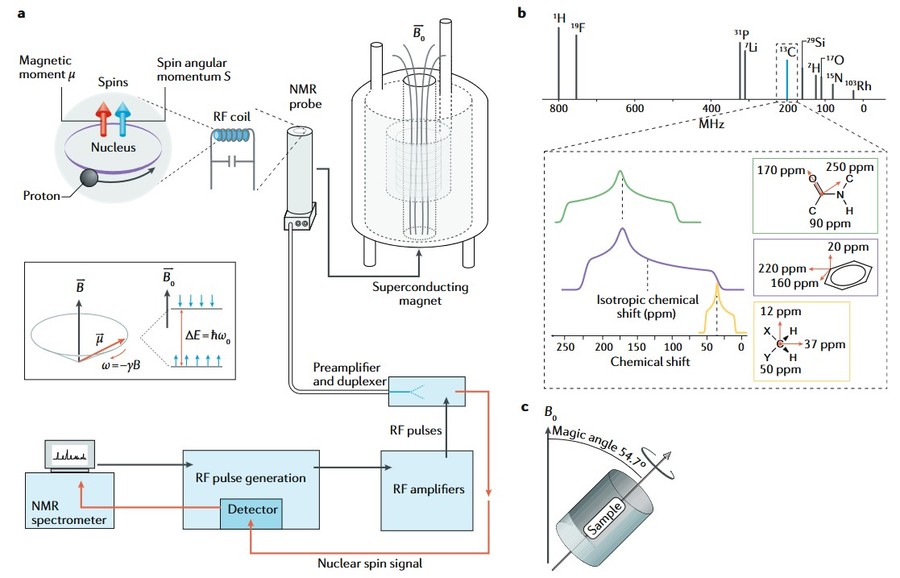
- NMR
- Particle Characterization
- Protein
- Protein Engineering
- Proteomics
- PTMs
- RNA
- Sample Preparation
- Structure
- Structure Prediction
- Target Proteins
- TEM
- XPS
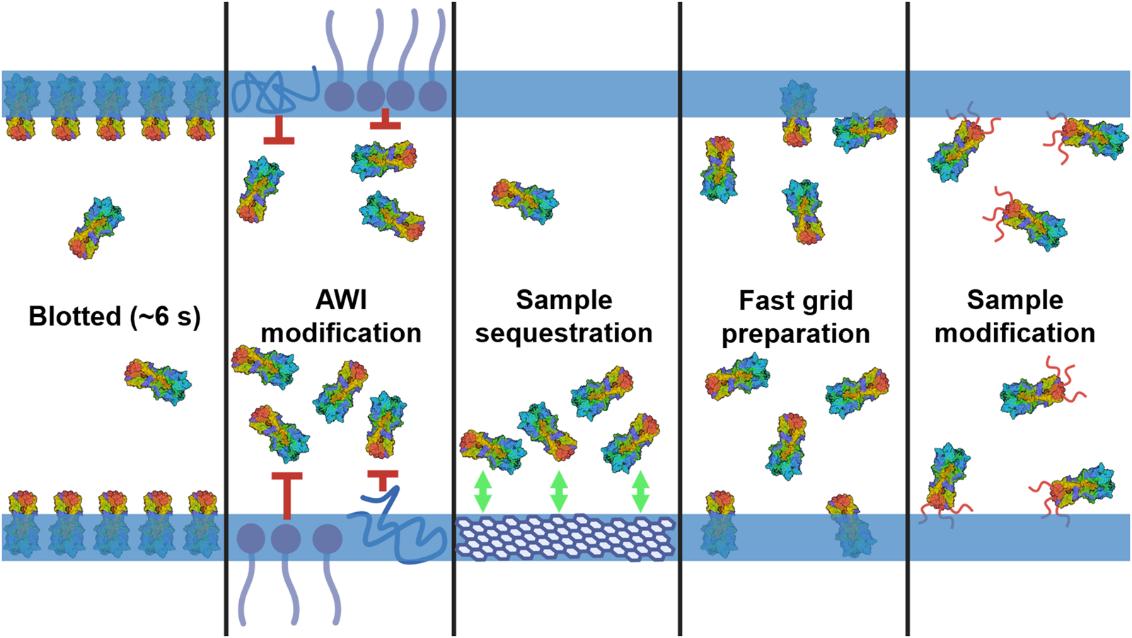
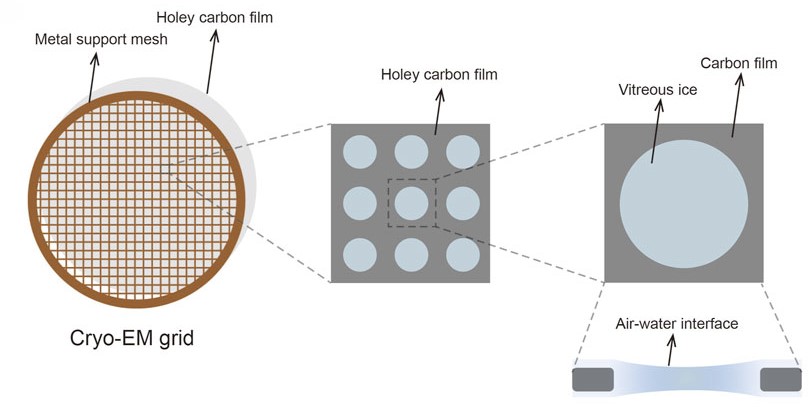
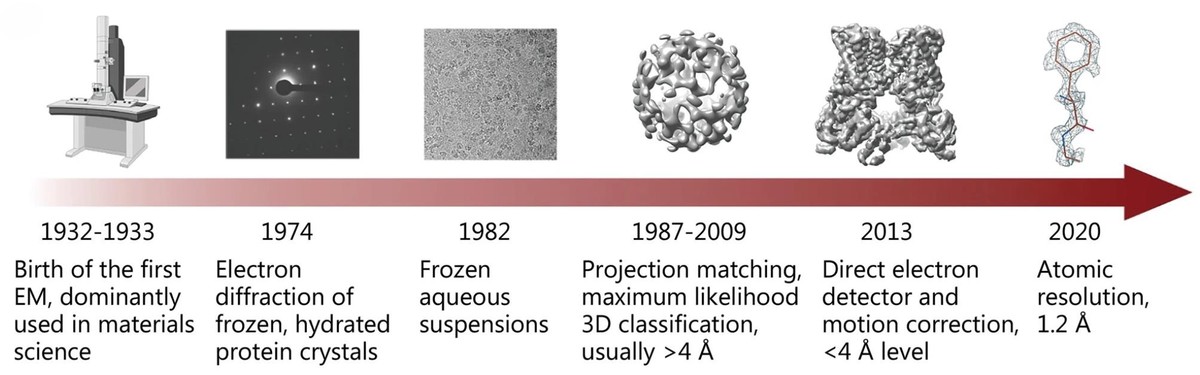
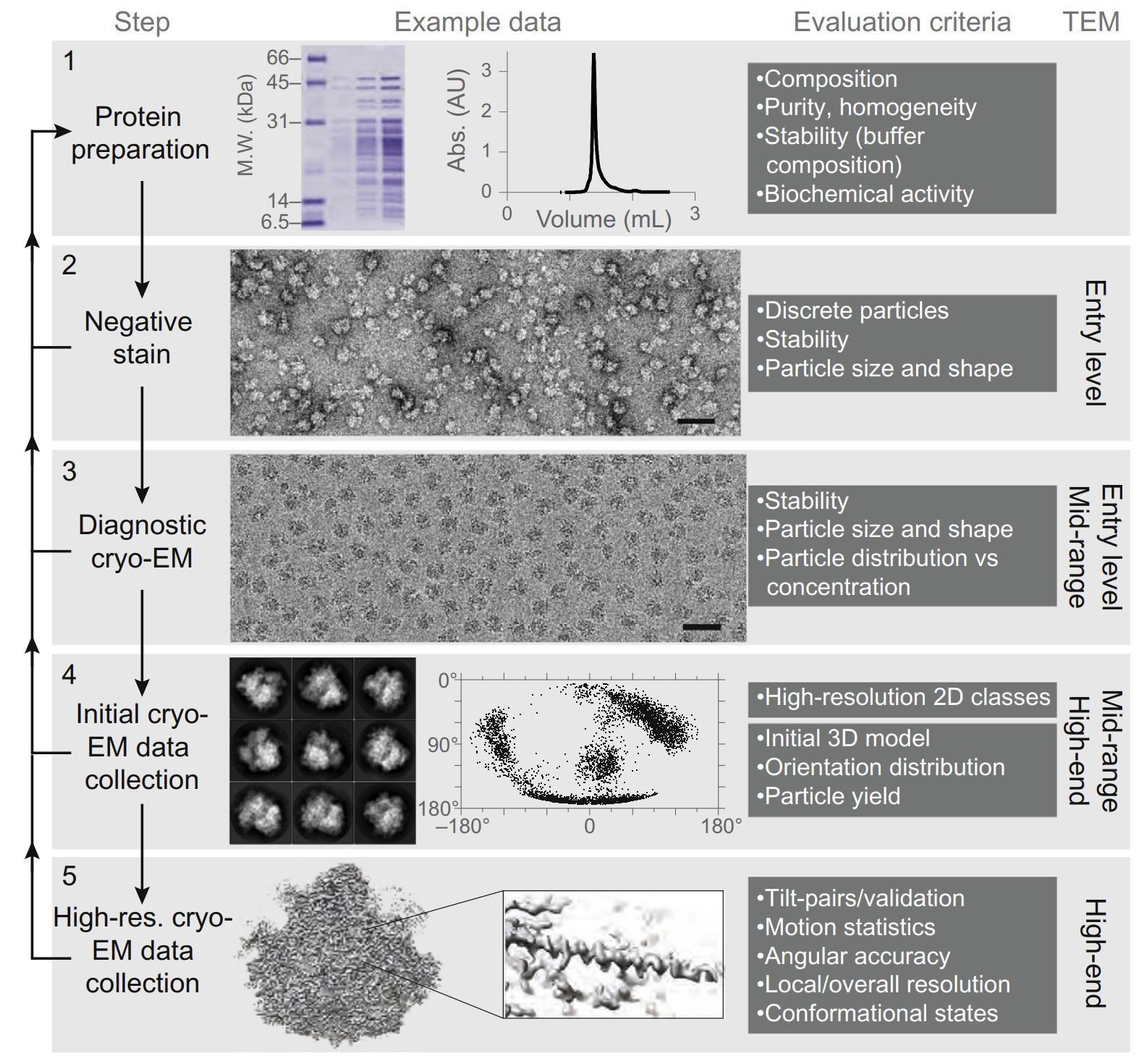
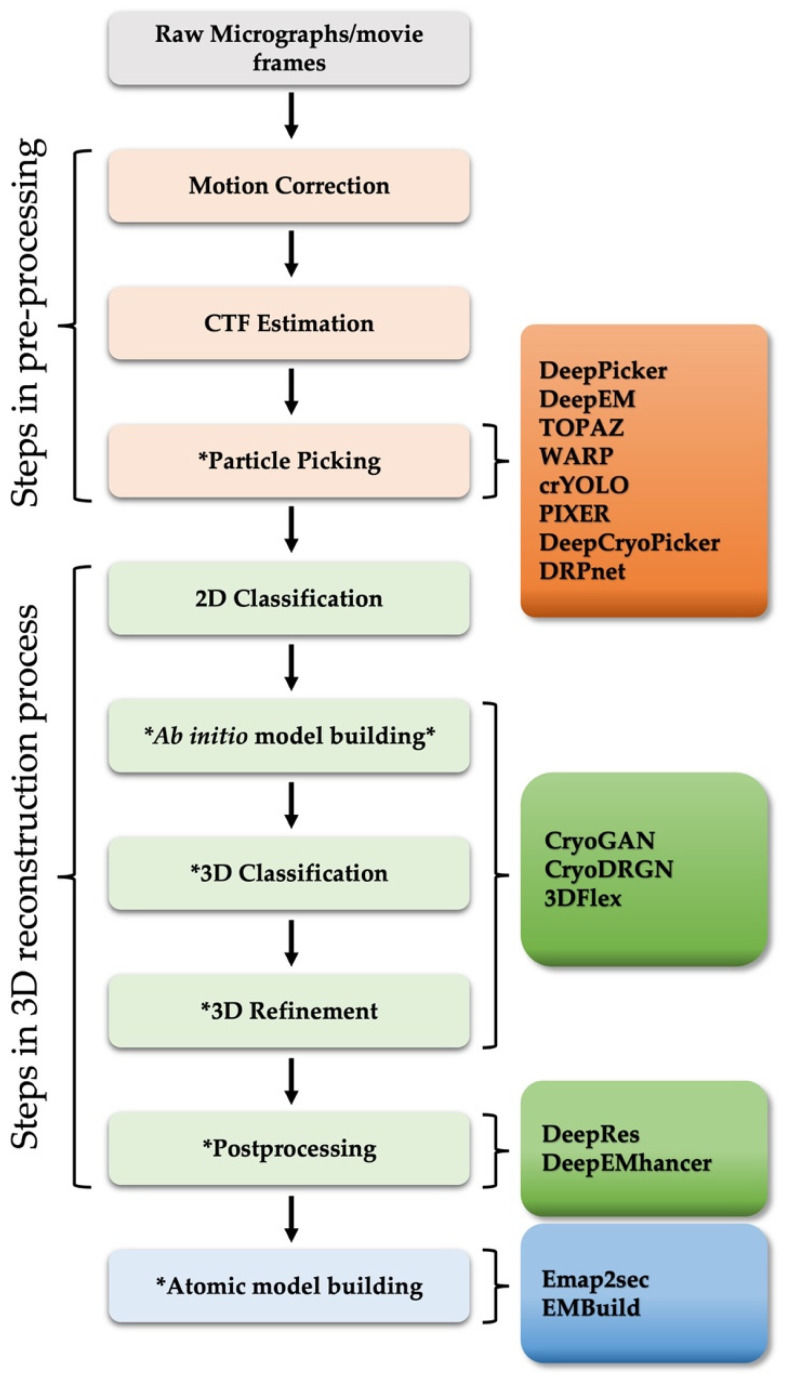
Cryo-EM Grid Preparation: A Comprehensive Guide to Sample Optimization
Structure
Cryo-EM
Sample Preparation
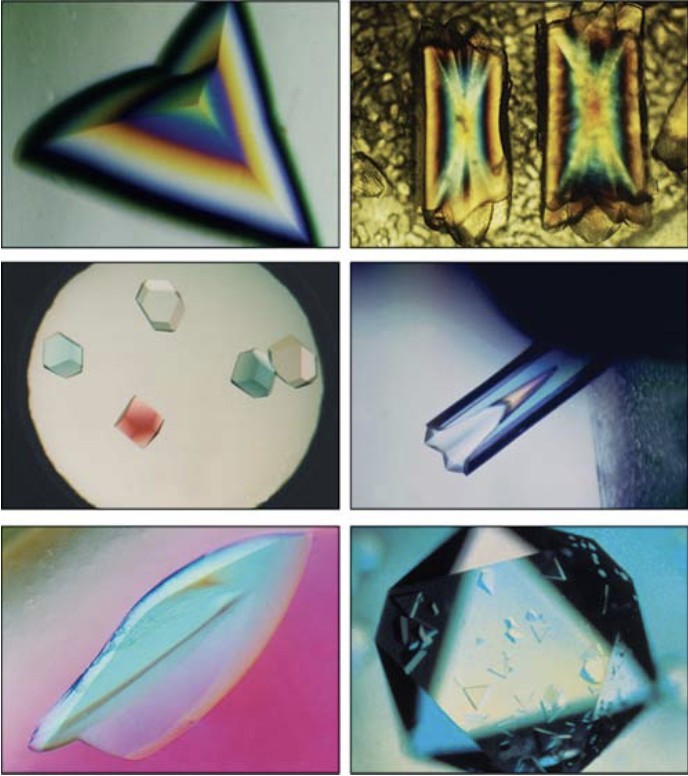
From Solution to Crystal: Mastering Protein Crystallization
Structure
Target Proteins
Crystallography

Common Problems in Protein X-ray Crystallography and How to Solve Them
Structure
Target Proteins
Crystallography
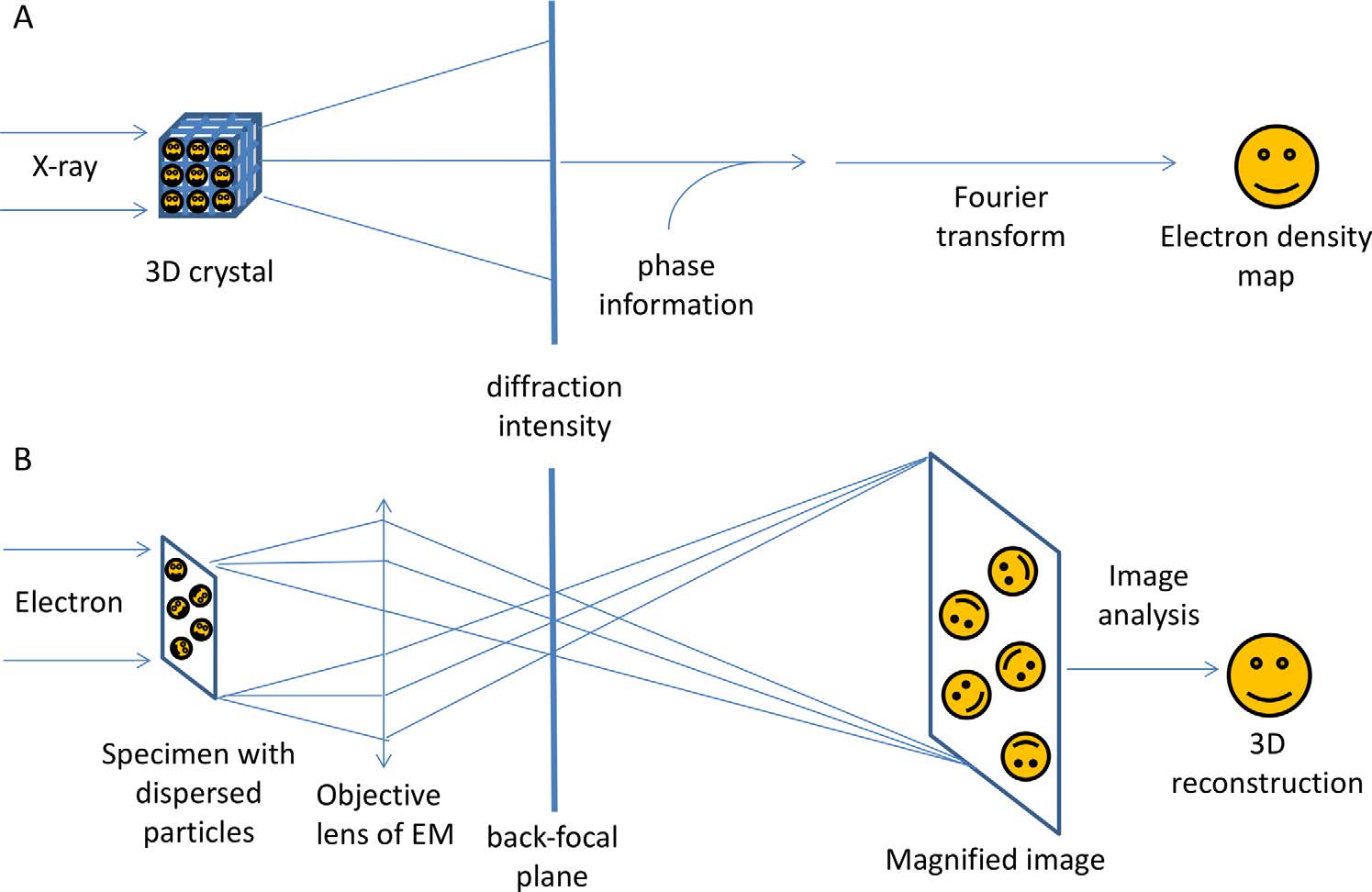
X-ray Crystallography vs Cryo-EM: Which is Best for Your Research?
Structure
Cryo-EM
Crystallography
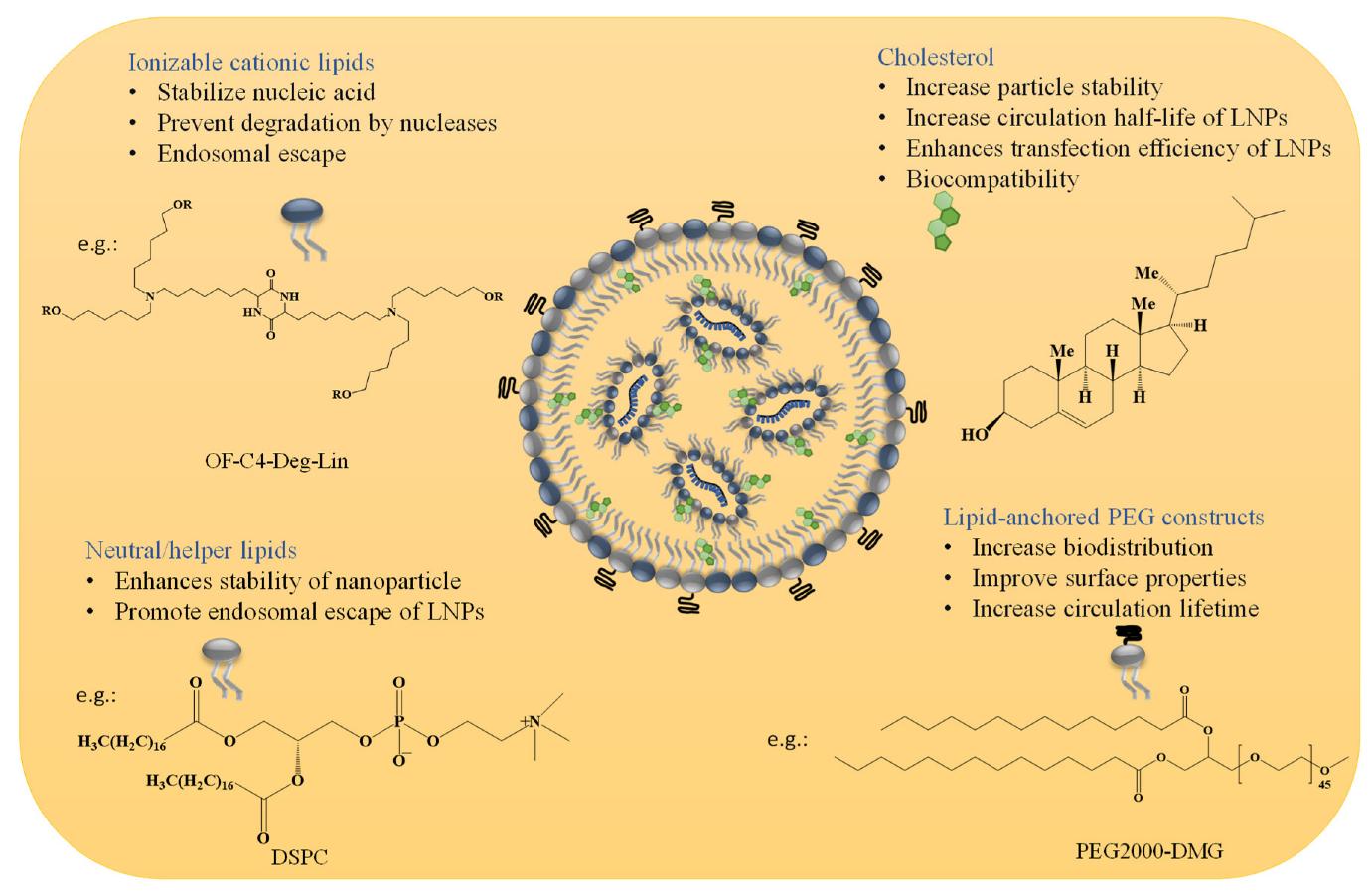
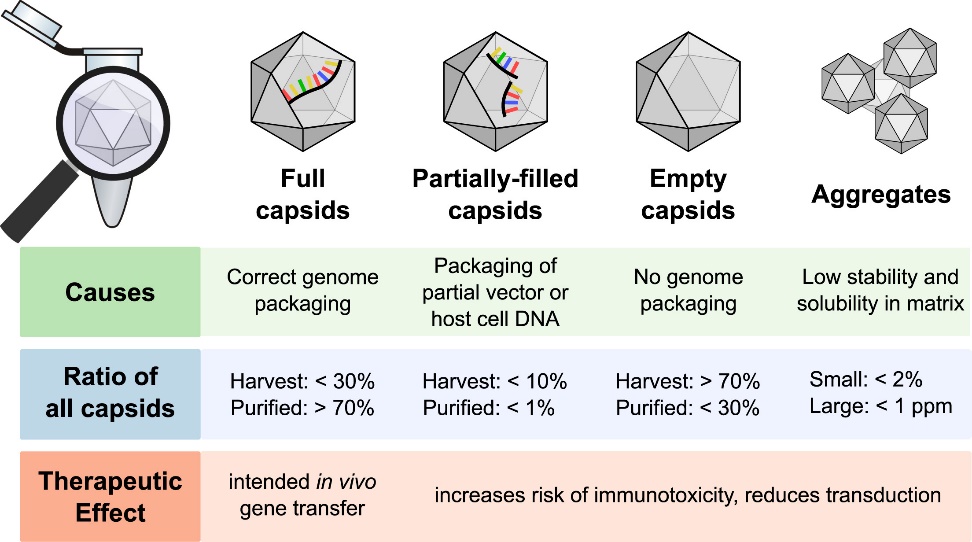
Cryo-EM Applications in Lipid Nanoparticle Characterization
Drug Development
Cryo-EM
Particle Characterization

Structural Studies of Hot Target Proteins: Techniques, Insights, and Challenges
Structure
Target Proteins
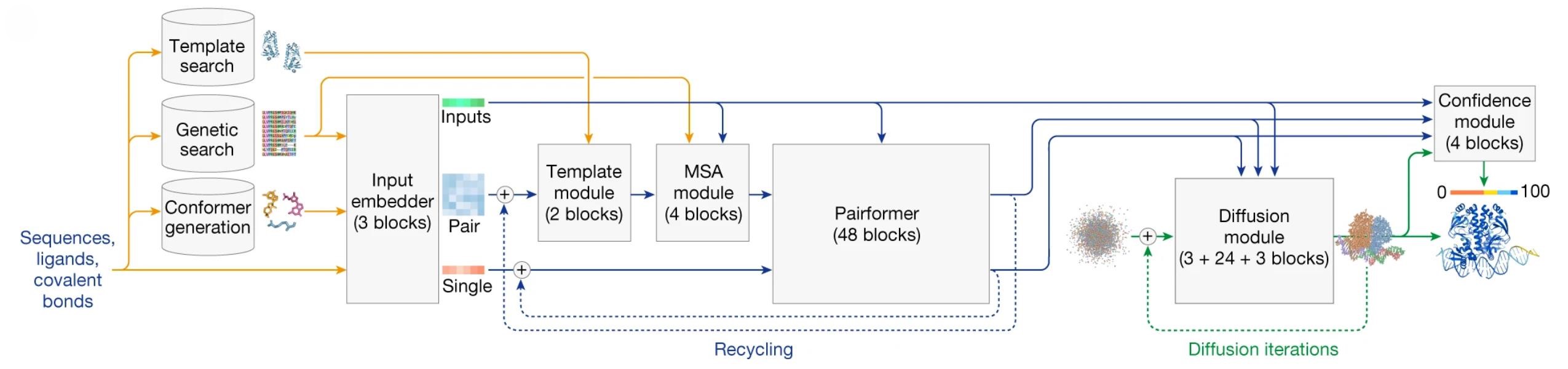
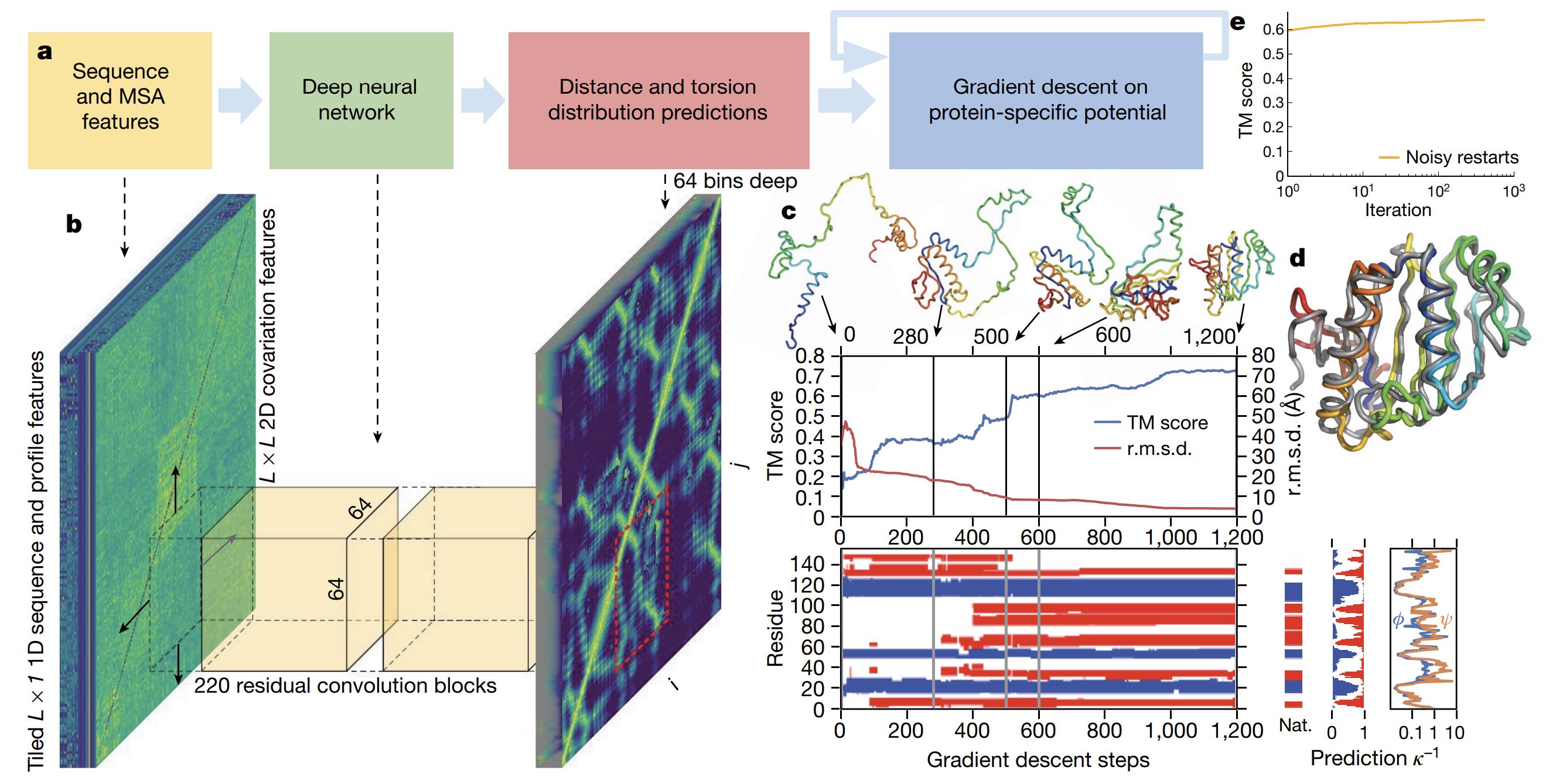
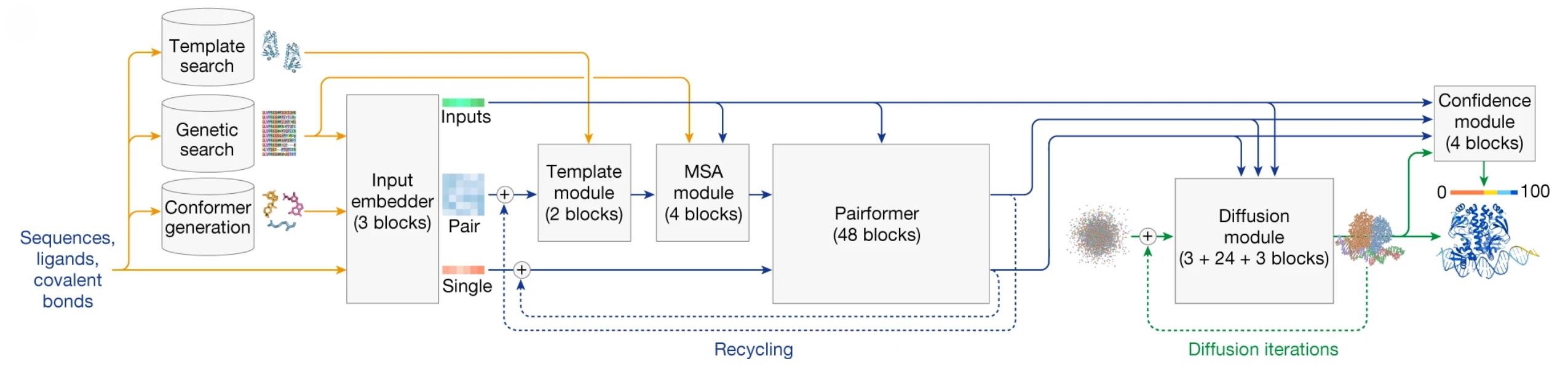
The Evolution of Protein Structure Prediction from Homology Modeling to AlphaFold
AI
Structure Prediction

Optimizing Protein Production and Purification for Crystallography
Structure
Target Proteins
Crystallography
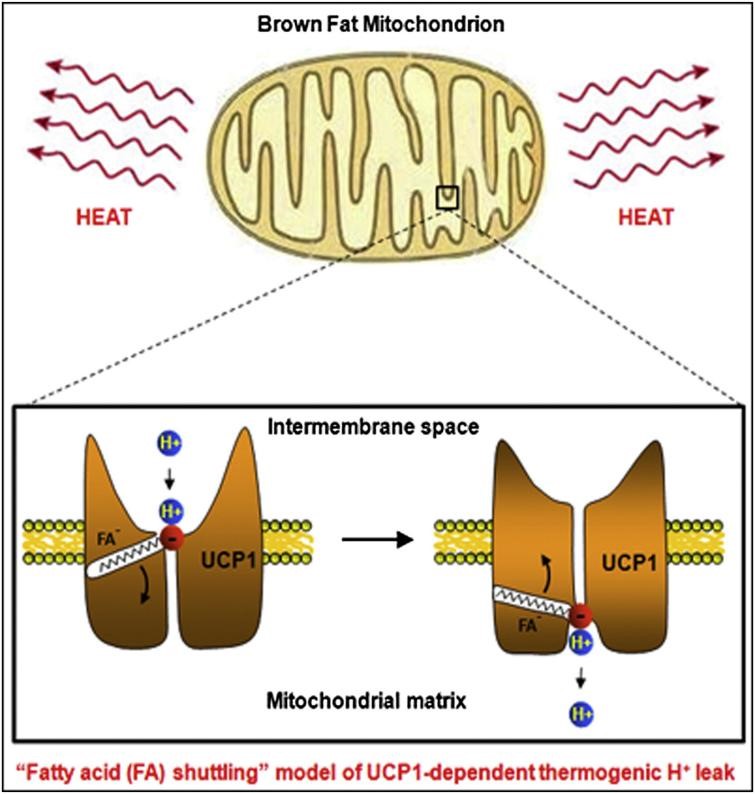
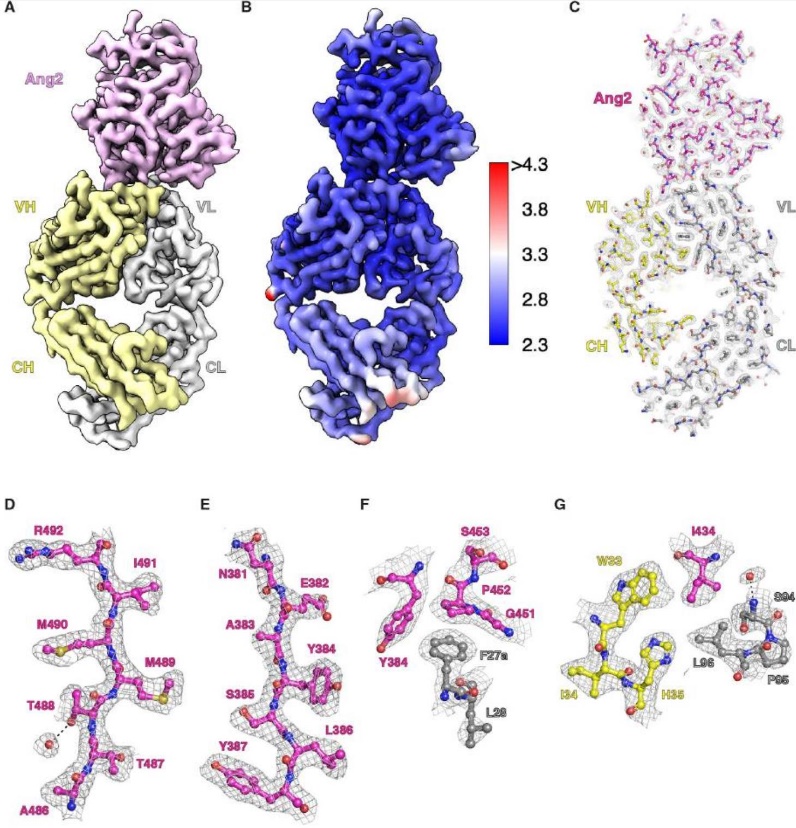
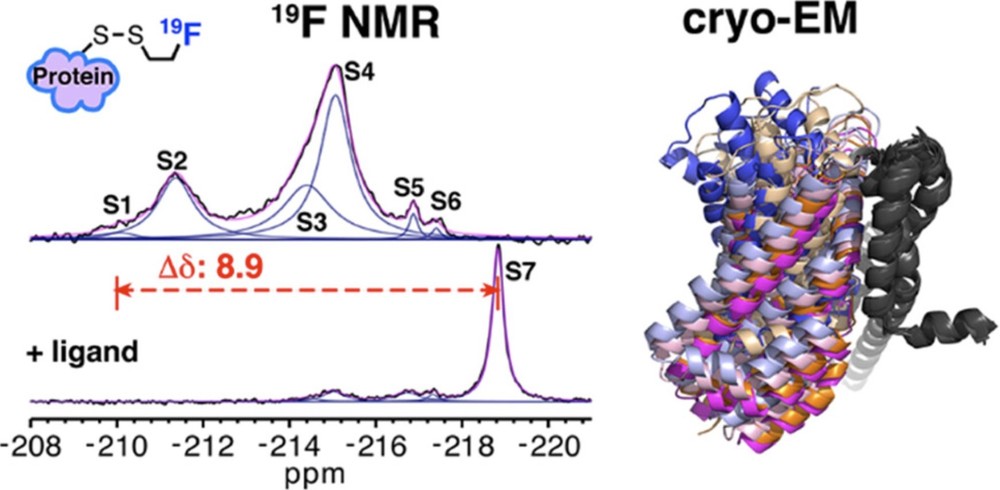
New Research: Cryo-EM Promotes the Research on UCP1 Working Mechanism
Structure
Target Proteins
Cryo-EM