Introduction to Protein Crystallization
Protein crystallization is a crucial technique in structural biology for determining the 3D structures of proteins. By revealing the atomic arrangement, scientists can better understand protein function and its role in biological processes. This technique is essential for both basic research and drug discovery, where high-quality crystals are needed for protein X-ray crystallography to generate accurate structural models.
The process involves organizing protein molecules into a well-ordered crystal lattice. The goal is to obtain high-diffraction-quality single crystals, which can be analyzed using X-ray crystallography to uncover atomic-level structures. Achieving this requires precise control over factors like pH, temperature, precipitating agents (e.g., polyethylene glycol, salts), and additives (such as ions or ligands) to induce crystal formation from supersaturated solutions.
This article will explore the key factors and methods involved in protein crystallization, focusing on the challenges and best practices to guide researchers in structural biology and drug discovery.
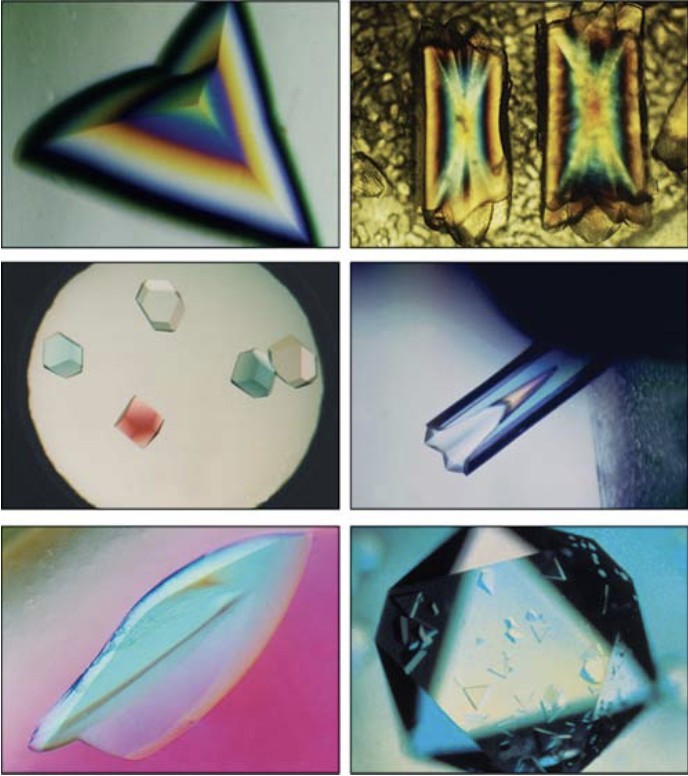 Figure 1. Examples of Various Protein Crystals. Images showcasing different types of protein crystals, highlighting variations in shape, size, and morphology. (McPherson A, et al., 2014)
Figure 1. Examples of Various Protein Crystals. Images showcasing different types of protein crystals, highlighting variations in shape, size, and morphology. (McPherson A, et al., 2014)
Basic Principles of Protein Crystallization Process
Protein Solution Preparation
The crystallization process starts with the preparation of a purified protein solution. Proteins must be carefully dissolved in a solvent that ensures they remain stable and homogeneous throughout the process. It's essential to purify the protein to remove any impurities that could interfere with crystal formation. Only once the protein solution is purified and stable can the crystallization process proceed.
Supersaturation and Its Importance
The first key stage in the crystallization process is supersaturation. Supersaturation occurs when the concentration of protein in solution exceeds its solubility, creating an unstable environment. This state promotes the aggregation of protein molecules, which is a necessary precursor to crystal formation. Maintaining a controlled level of supersaturation is crucial for the success of subsequent stages. Too high a supersaturation can cause unwanted precipitation, while too low can hinder the formation of well-ordered crystals.
Nucleation and Seed Formation
Once supersaturation is achieved, nucleation occurs, where small "seeds" of crystals form. These seeds act as the foundation for larger crystal growth. Nucleation can occur spontaneously, but researchers often use seeding techniques to introduce pre-formed crystal seeds into the solution to help initiate crystal formation. Proper control of nucleation is vital, as too many seeds can result in many small crystals, while too few can prevent the formation of high-quality crystals altogether.
Crystal Growth and Formation
In the crystal growth phase, the small nucleated seeds begin to grow into larger, well-ordered crystals. This phase depends on the slow diffusion of precipitating agents or other factors that maintain the supersaturated environment. As the crystals grow, their internal structure becomes increasingly ordered, leading to larger and more stable crystals. These well-formed crystals are essential for techniques like X-ray crystallography, where high-quality diffraction data is needed to determine the protein's atomic structure.
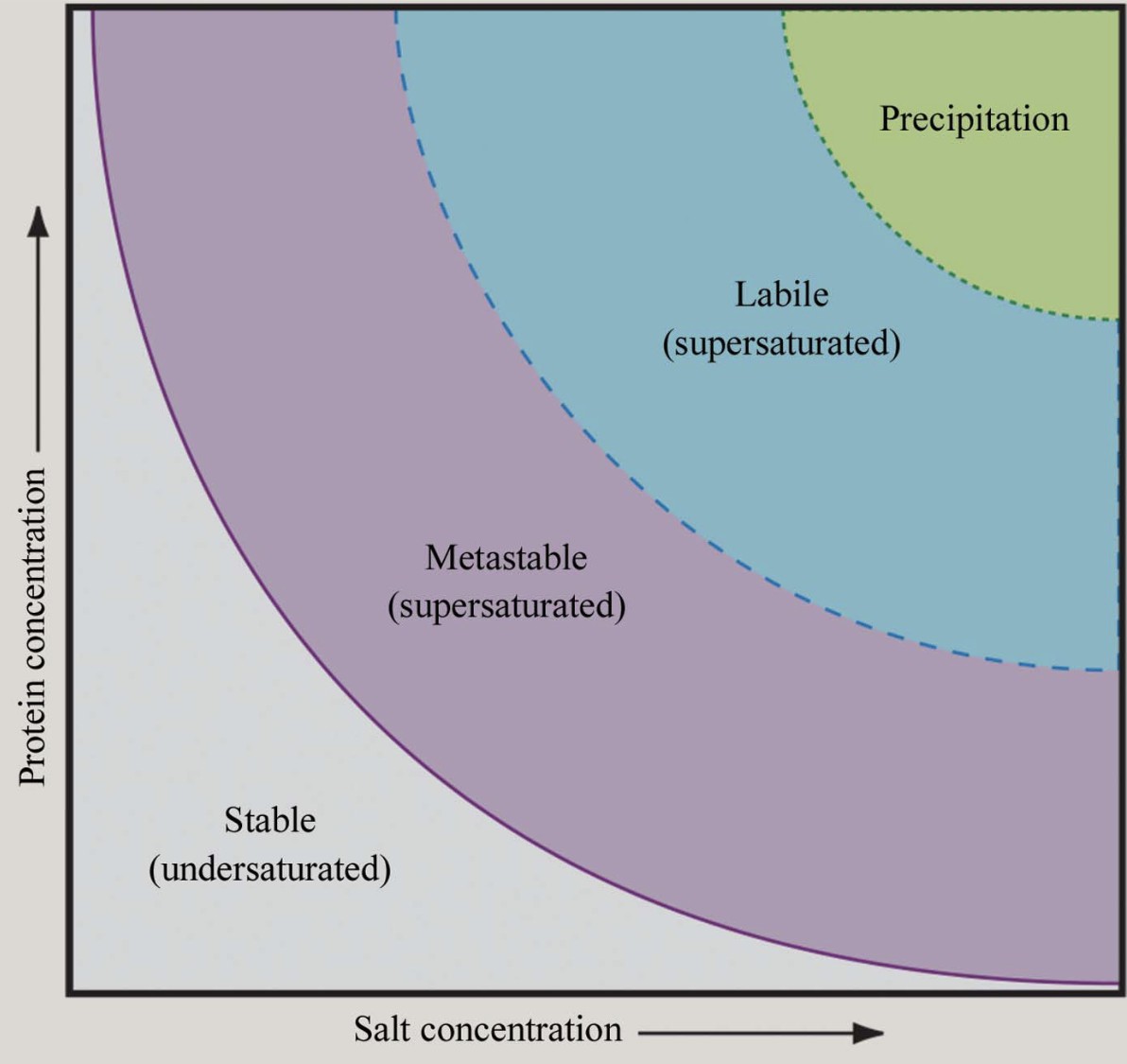 Figure 2. Phase Diagram for Protein Crystallization. Solubility phase diagram illustrating undersaturation, supersaturation, and crystallization regions, including metastable, labile, and precipitation zones. (McPherson A, et al., 2014)
Figure 2. Phase Diagram for Protein Crystallization. Solubility phase diagram illustrating undersaturation, supersaturation, and crystallization regions, including metastable, labile, and precipitation zones. (McPherson A, et al., 2014)
Key Factors Affecting Protein Crystallization
Protein crystallization is a highly complex process influenced by multiple factors. The success of crystallization depends on the precise balance of conditions and factors affecting the protein solution, temperature, pH, additives, crystallization methods, and environmental factors.
1. Solution Conditions
A. Precipitants Type and Concentration
The choice of precipitants plays a significant role in protein crystallization. Common precipitants include:
- Salts (e.g., ammonium sulfate, sodium chloride) reduce protein solubility through a process known as "salting out," in which proteins aggregate due to the lowered solubility in the presence of salts. This follows the Hofmeister series, which predicts the effects of different salts on solubility.
- Polyethylene glycol (PEG), especially high molecular weight PEG (e.g., PEG 8000), is used to induce crystallization by excluding volume effects. PEG is beneficial for fine-tuned control and minimal side effects.
- Organic solvents (e.g., MPD) lower the dielectric constant of the solution, which can promote crystallization, but may also risk denaturing proteins.
B. Protein Concentration
A typical starting concentration is around 10 mg/ml but should be adjusted based on protein solubility and stability. High concentrations might lead to aggregation, while too low a concentration may hinder nucleation and crystal formation.
C. Salt Concentration and Ion Strength
Salt concentration is directly related to the protein's charge density. If the solution pH deviates from the protein's isoelectric point (pI), higher salt concentrations are necessary to neutralize electrostatic repulsion between protein molecules, facilitating crystal growth.
D. Buffer Selection
Common buffers such as Hepes and Tris are used to maintain the pH of the solution. Buffer concentrations typically range from 10-50 mM. It's essential to choose a buffer that does not interfere with crystal formation and avoid buffer systems that may cause salt crystal contamination.
2. Temperature Control
Temperature affects both the thermodynamic and kinetic aspects of protein solubility. Higher temperatures may increase solubility (entropy-driven) or reduce it (enthalpy-driven), affecting the crystallization process. Standard experimental temperatures are 4°C or 20°C, but newer temperature-control technologies offer finer adjustments to optimize crystal morphology.
3. Optimizing pH for Crystallization
A. Relationship between pH and Isoelectric Point (pI)
Acidic proteins (pI < 7) often crystallize best when the pH is 0-2.5 units above the pI. Basic proteins (pI > 7) tend to crystallize at a pH 0.5-3 units below their pI. Adjusting the pH within these ranges optimizes protein solubility and crystallization.
B. Effect of pH on Metal Coordination
Changes in pH can alter metal-binding sites on proteins, affecting their crystallization. For example, S100B protein binds zinc differently at pH values of 6.5 and 9.0. Understanding these variations is crucial when crystallizing metal-binding proteins.
4. Role of Additives in Protein Crystallization
A. PEG and Salt Synergistic Effects
The addition of salts like 0.5 M NaCl increases the cloud point of PEG 3350 by approximately 10°C, expanding the window of crystallization conditions.
B. Specific Additives
Detergents, such as DDM, maintain membrane protein solubility, essential for successful crystallization.
Sugars (e.g., sucrose) can act as stabilizers, reducing surface tension and enhancing crystal quality.
C. Cross-precipitation Effects (CIP)
Using combinations of selective additives, such as low-concentration alcohols with salts, can promote the formation of specific crystal forms, opening up novel crystallization strategies.
5. Crystallization Method Selection
A. Vapor Diffusion Method
This method controls the diffusion of water molecules, gradually reaching supersaturation and facilitating crystal formation. It's effective for exploring a broad range of crystallization conditions but is typically slower.
B. Microbatch Method
In this method, protein and precipitant are mixed directly under fixed conditions. It's faster for screening but offers less flexibility compared to other methods.
C. Microfluidic Technology
Microfluidic techniques generate uniform droplets and allow real-time monitoring of crystallization conditions. Combining microfluidics with machine learning algorithms significantly enhances the success rate of crystal formation.
6. Environmental Factors
A. Humidity Control
High humidity environments can interfere with the vapor diffusion process. To ensure stable conditions, sealed systems (e.g., oil seals) are used to prevent fluctuations in humidity.
B. Mechanical Stability
Mechanical vibrations during crystallization can disrupt the formation of nucleation centers. To avoid this, anti-vibration tables are commonly used in laboratories to minimize disturbance during critical stages of crystallization.
Protein Crystallization Methods
Protein crystallization techniques are diverse, each with unique advantages and specific applications. Below is a detailed breakdown of several key methods: Vapor Diffusion, Batch Crystallization and Dialysis, and Microbatch and Free Interface Diffusion.
Vapor Diffusion Techniques
Vapor diffusion is one of the most widely used protein crystallization methods, typically implemented via Hanging Drop or Sitting Drop techniques. The principle behind vapor diffusion is based on the equilibrium of vapor diffusion, where the concentration of the protein and precipitant in the droplet gradually increases, leading to supersaturation and crystal formation.
Vapor Diffusion Process
- Drop Preparation: A droplet containing a mixture of protein solution and precipitant is placed inside a sealed chamber.
- Vapor Equilibrium: The chamber's reservoir contains a high concentration of precipitant, and through vapor diffusion, the solvent content in the droplet is reduced, gradually increasing the degree of supersaturation.
- Inducing Phase Change: Once the supersaturation reaches a critical point, protein molecules begin to nucleate and grow into crystals.
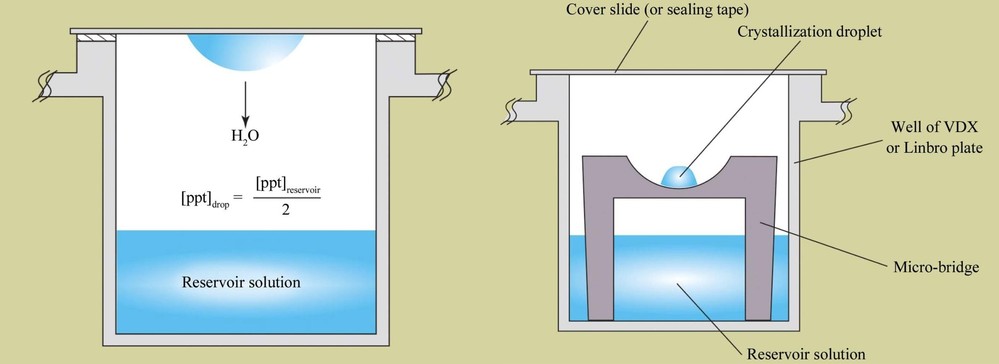 Figure 3. Hanging-Drop and Sitting-Drop Vapor Diffusion Methods. Schematic comparison of hanging-drop (left) and sitting-drop (right) vapor-diffusion methods for protein crystallization, illustrating equilibration processes. (McPherson A, et al., 2014)
Figure 3. Hanging-Drop and Sitting-Drop Vapor Diffusion Methods. Schematic comparison of hanging-drop (left) and sitting-drop (right) vapor-diffusion methods for protein crystallization, illustrating equilibration processes. (McPherson A, et al., 2014)
Automation and Image Classification
Recent advancements have integrated high-throughput screening (HTS) and machine learning to enhance the efficiency of vapor diffusion.
- Dynamic Programming Curve Tracking Algorithm: Used for automatically detecting droplet boundaries and extracting statistical features (e.g., roundness, gradient intensity). The images are then classified into categories like "Empty," "Clear," "Precipitate," "Microcrystals," and "Crystals," achieving an accuracy of about 85%.
- Deep Convolutional Neural Networks (CNNs): By training on large image datasets (e.g., 150,000 crystallization images), CNNs provide real-time classification of crystallization states, excelling in X-ray diffraction image analysis.
Advantages and Disadvantages of Vapor Diffusion Method
- Advantages: Simple to operate, suitable for high-throughput screening, and offers precise control over supersaturation gradients.
- Disadvantages: Sensitive to initial conditions, easily affected by environmental fluctuations (e.g., temperature changes), and typically requires a long equilibration period (from days to weeks).
Application Scenarios of Vapor Diffusion Method
- Initial Screening: Ideal for rapidly exploring a wide range of chemical conditions (e.g., different combinations of precipitants).
- Structural Biology Laboratories: Commonly used for routine crystal growth and optimization.
Batch Crystallization and Dialysis
1. Batch Crystallization
Batch crystallization occurs in a closed system where oversaturation is achieved by adjusting temperature or solvent composition.
- Cooling Control: Oversaturation is controlled by applying linear or nonlinear cooling curves. For example, potassium dihydrogen phosphate (KDP) crystals can be optimized by adjusting the cooling rate.
- Seed Crystallization: Adding seed crystals can suppress spontaneous nucleation and improve uniformity in crystal size. Research shows that using an optimal seed amount (e.g., for lysozyme crystallization) prevents the formation of fine crystals.
Kinetic Models and Optimization
- Dynamic Optimization Models: These models combine thermodynamic equations with heat transfer control to maximize crystal size. For example, in potassium nitrate crystallization, the average crystal size increased by 48%.
- Low-Cost Monitoring: Crystal kinetics can be analyzed using absorbance measurements, eliminating the need for expensive light-scattering instruments.
Advantages and Disadvantages of Batch Crystallization
- Advantages: Simple operation, cost-effective, and suitable for scaling up in industrial applications.
- Disadvantages: Potential for wide crystal size distributions, requiring precise control of cooling rates and seed quantities.
2. Dialysis
Dialysis involves using a semi-permeable membrane to separate the protein solution from the precipitant, allowing gradual adjustment of concentration to reduce nucleation sites. The protein solution is placed in a dialysis bag, which is then immersed in an external solution containing precipitant. Diffusion equilibrium adjusts the concentration of both the protein and precipitant.
Application Scenarios of Dialysis
This method is especially beneficial for proteins sensitive to shear forces or when microgravity conditions are used to improve crystal quality.
Microbatch and Free Interface Diffusion
1. Microbatch Crystallization
Microbatch crystallization involves small-volume operations, often carried out in an oil phase (such as paraffin oil) to prevent evaporation. The droplet volume can be as small as 10-100 nL, making it highly efficient for screening.
- High-Throughput Screening: Microfluidic technologies like SlipChip allow for the testing of various mixing ratios simultaneously. A 10 μL protein solution can be used to conduct up to 1,300 trials.
Advantages and Disadvantages of Microbatch Crystallization
- Advantages: Minimal sample usage, ideal for rare proteins, and fast operation.
- Disadvantages: The oil phase may interfere with crystallization, requiring careful optimization of interface conditions.
2. Free Interface Diffusion (FID)
FID relies on the direct contact between the protein and precipitant, forming a concentration gradient at the interface, simulating different areas of a phase diagram.
- Composite Method (FID + Microbatch): By combining FID with microbatch techniques on a SlipChip device, different diffusion equilibrium times and mixing ratios can be tested simultaneously, improving successful crystallization conditions by 30% compared to using each method individually.
Application Scenarios of Free Interface Diffusion
- Membrane Protein Crystallization: This method has been successful in the crystallization of bacterial membrane proteins, such as DHFR/TS, yielding a high-resolution structure at 1.95 Å.
- Real-Time Optimization: Allows for the screening and optimization of crystallization conditions within a single experiment, reducing false negatives.
Comparative Analysis of Protein Crystallization Techniques
| Method | Applicable Stage | Sample Consumption | Automation Potential | Crystal Quality Control |
|---|---|---|---|---|
| Vapor Diffusion | Initial Screening and Optimization | Moderate | High | Dependent on Environmental Stability |
| Batch Crystallization | Industrial Scale-up | High | Moderate | Requires Precise Control of Cooling/Nucleation |
| Micro-Batch Crystallization | High-Throughput Initial Screening | Extremely Low | High | Potentially Affected by Oil Phase Interference |
| Free Interface Diffusion | Complex Condition Exploration | Low | High | Flexible Gradient Control |
Crystal Optimization Techniques
Seeding and Microseeding Methods
Seeding techniques involve the introduction of pre-formed crystals into a fresh solution to stimulate further crystal growth. Microseeding, a more refined approach, uses tiny seed crystals to initiate nucleation, significantly improving the probability of obtaining high-quality protein crystals. These methods are particularly useful for overcoming nucleation barriers and enhancing reproducibility in crystallization experiments.
The standard procedure includes the following steps:
- Seed Preparation: Initial crystals are fragmented and serially diluted (e.g., 10-fold dilution steps) to produce micron-sized seed crystals.
- Seed Introduction: The prepared seed solution is introduced into a supersaturated protein solution, often using techniques such as counter-diffusion in capillaries to achieve uniform distribution.
Key application cases are as follows:
- Resolving Twinning Issues: In the case of LigM protein, microseeding combined with reservoir composition optimization successfully eliminated twinning problems, ultimately yielding high-resolution crystals (<2.0 Å).
- Facilitating Crystallization of Low-Solubility Proteins: For proteins with low solubility or limited availability (e.g., lysozyme), microseeding circumvents nucleation barriers and significantly improves crystallization success rates.
Seeding and microseeding have become indispensable tools in modern protein crystallography, enabling researchers to refine crystallization conditions and obtain diffraction-quality crystals.
Chemical Modifications and Deglycosylation
Chemical modifications, including deglycosylation (removal of carbohydrate groups), can improve crystallization by enhancing protein homogeneity or altering the protein's conformation for better lattice formation.
Role of Deglycosylation
Glycosylation often introduces heterogeneity that hinders crystallization by causing structural variability. Removing glycans can improve crystal formation by reducing disorder and creating a more uniform surface charge distribution. For example, trifluoromethanesulfonic acid (TFMS) has been used to efficiently strip glycans from glycoproteins, significantly enhancing the crystallization success of proteins such as antibodies.
Other Chemical Modifications
- Disulfide Bond Reduction: Treating proteins with β-mercaptoethanol can break intermolecular disulfide bonds, preventing unwanted cross-linking—an effective strategy for cysteine-rich proteins.
- Proteolytic Trimming: Limited proteolysis with specific proteases removes flexible N-terminal or C-terminal regions, increasing protein rigidity and improving crystallization efficiency.
By strategically applying these chemical modifications, researchers can optimize protein samples for crystallization, overcoming structural challenges that often impede high-resolution structure determination.
Complex Formation with Ligands and Co-factors
Crystallization success can be significantly improved by forming complexes between proteins and ligands, co-factors, or antibodies. These molecular interactions help stabilize the protein's conformation, reduce flexibility, and promote better lattice packing, ultimately enhancing crystallization efficiency.
Stabilizing Effects of Ligand Binding
Ligand binding can lock a protein into a specific conformation, reducing conformational heterogeneity and facilitating ordered crystal growth. For example, the antimalarial drug 8-aminoquinoline (+PQ) stabilizes human monoamine oxidase (MAO) by interacting with FAD (flavin adenine dinucleotide) and key residues (Y444, I325, etc.), leading to improved crystal quality.
The Critical Role of Co-factors
Co-factors such as FAD and metal ions are essential for protein structure stabilization, often playing a key role in maintaining quaternary structure integrity. Studies have shown that co-factor-bound proteins exhibit higher structural conservation within homologous complexes due to the spatial constraints imposed by their binding sites.
Technical Strategies for Complex Formation
- Co-crystallization Optimization: Adding substrate analogs or competitive inhibitors into crystallization conditions can induce a specific and stable conformation.
- Metal Ion Chelation: Magnesium ions (Mg²⁺), for instance, stabilize the active conformation of ATP-binding proteins, facilitating successful crystallization.
By leveraging ligand and co-factor interactions, researchers can enhance protein stability and improve crystallization outcomes.
Select Service
Related Reading
Protein Crystallization Protocols
Step-by-Step Guide to Setting Up Crystallization Experiments
Setting up a protein crystallization experiment requires careful optimization of multiple parameters, including protein concentration, buffer composition, and precipitants. The process often involves iterative adjustments to achieve optimal conditions for crystal growth. Below is a detailed protocol for setting up crystallization experiments, particularly using the hanging drop vapor diffusion method for membrane proteins.
1. Sample Preparation
- Protein Purification and Concentration: Purify the protein and concentrate it to 5–50 mg/mL, adjusting based on the protein's solubility and stability.
- Buffer Exchange and Dialysis: Remove unwanted salts and small molecules through dialysis or size-exclusion chromatography to ensure a well-defined buffer environment.
2. Crystallization Plate Setup
- Reservoir Preparation: Pipette 500 μL of crystallization solution (containing precipitant, buffer, and salts) into the reservoir well of a crystallization plate.
- Drop Formation: Mix 1 μL of protein solution with 1 μL of reservoir solution on a glass cover slip. Carefully invert and seal the cover slip over the reservoir well to initiate vapor diffusion.
3. Incubation and Monitoring
- Controlled Environment: Incubate at a stable temperature of 18-20°C, minimizing vibrations.
- Daily Microscopy Checks: Examine drops under a microscope (40× magnification or higher) to detect early crystal formation. Polarized light microscopy can be used to identify birefringent crystals.
4. Optimization and Scale-up
- Parameter Adjustments: Once initial crystals appear, fine-tune conditions: Adjust PEG or precipitant concentration by ±5%. Modify pH gradient in 0.2-0.5 unit increments.
- Grid Screening Approach: Use a 2D matrix screen (e.g., precipitant concentration vs. pH) to systematically refine crystallization conditions.
5. High-Throughput Screening Strategies
- Sparse Matrix Screening: Utilize pre-designed crystallization screens to efficiently sample a wide chemical space.
- Phase Diagram-Guided Selection: Apply microfluidic techniques to measure protein solubility curves, allowing precise identification of supersaturation zones for targeted crystallization trials.
By following these structured steps and refining conditions iteratively, researchers can increase the likelihood of obtaining well-diffracting protein crystals suitable for structural studies.
Troubleshooting Common Issues in Protein Crystallization
Protein crystallization can be challenging, with common issues including lack of crystal formation, amorphous precipitation, poor crystal quality, or crystal dissolution. Effective troubleshooting often requires fine-tuning experimental parameters or employing alternative approaches. Below are common problems and their solutions:
| Issue | Cause | Solutions |
|---|---|---|
| No Crystal Formation | - Insufficient supersaturation - High nucleation energy barrier |
- Increase precipitant concentration (e.g., PEG +2-5%) - Introduce seed crystals (e.g., microseeding, nanoparticles) - Adjust pH toward protein's isoelectric point (pI) - Change buffer system (e.g., Tris → MES) |
| Amorphous Precipitation or Cloudiness | - Excessive supersaturation - Uncontrolled aggregation |
- Reduce precipitant concentration - Apply gradient approach (e.g., stepwise PEG addition) - Add stabilizers (e.g., 0.1-1% glycerol, 5 mM DTT) - Adjust incubation temperature (4°C vs. 20°C) |
| Poor Crystal Quality (Small, Twinned, Weak Diffraction) | - Structural disorder - Inhomogeneous nucleation |
- Apply microseeding for controlled crystal growth - Screen additives (e.g., 2-8 mM spermine, Ca²⁺) - Use anaerobic conditions (inert gas environment) for oxygen-sensitive proteins |
| Crystal Dissolution | - Sudden solution changes - Mechanical disturbances |
- Gradually adjust reservoir precipitant concentration (PEG +0.5% every 24 hours) - Use silicone oil overlays to minimize evaporation |
Evaluation of Crystallization Results
Assessing crystallization outcomes is crucial to determine the quality and suitability of crystals for structural analysis. The evaluation process involves both visual inspection and diffraction testing to ensure optimal crystal properties.
Initial Screening Assessment
Crystals are first examined under a microscope to assess their morphology, size, and transparency. Well-formed single crystals are preferred over twinned or clustered formations. Ideally, crystals should be larger than 50 µm and highly transparent, as these characteristics often correlate with better diffraction quality. We now offer custom trace fluorescent labeling crystallization as a powerful technique to enhance the identification and optimization of macromolecular crystals.
Diffraction Testing
X-ray diffraction analysis, typically performed at synchrotron facilities with cryocooled crystals (100 K), determines resolution and lattice consistency. Crystals with a resolution better than 3 Å are generally suitable for structural studies. Lattice parameters are analyzed to confirm reproducibility and ensure the crystal's suitability for data collection.
In conclusion, protein crystallization is a crucial step in determining the three-dimensional structure of proteins, involving a complex process from solution preparation to crystal optimization. Various factors, including solution conditions, temperature, pH, and additives, influence crystal growth, and optimizing these parameters is essential for success. Different crystallization methods have unique advantages, making method selection and refinement critical for obtaining high-quality crystals.
At Creative Biostructure, we offer comprehensive, one-stop protein x-ray crystallography services, from protein expression and crystallization screening to optimization and structure analysis. We also provide customized solutions for individual steps based on your specific needs. Contact us today to accelerate your research!
References
- Chayen N E, Saridakis E. Protein crystallization: from purified protein to diffraction-quality crystal. Nature Methods. 2008, 5(2): 147-153.
- Krauss I R, Merlino A, Vergara A, et al. An overview of biological macromolecule crystallization. International Journal of Molecular Sciences. 2013, 14(6): 11643-11691.
- McPherson A, Gavira J A. Introduction to protein crystallization. Structural Biology and Crystallization Communications. 2014, 70(1): 2-20.
- Chaurasiya N D, Liu H, Doerksen R J, et al. Enantioselective interactions of anti-infective 8-aminoquinoline therapeutics with human monoamine oxidases A and B. Pharmaceuticals. 2021, 14(5): 398.
- Trubitsin B V, Milanovsky G E, Mamedov M D, et al. The interaction of water-soluble nitroxide radicals with Photosystem II. Applied Magnetic Resonance. 2022: 1-15.
- Ghosh R. Membrane-Based Micro-Volume Dialysis Method for Rapid and High-Throughput Protein Crystallization. Processes. 2023, 11(7): 2148.