Batch Screening for Crystal Production
Growth of high-quality crystals is a major obstacle in many structural investigations. In recent years, the techniques for screening crystals have improved dramatically. Creative Biostructure is pioneer in the field of crystals screening, such as peptide crystallization and has led many successful campaigns to produce the crystals in small and large scale.
Besides for macromolecular structural study, a crystalline formulation has some advantages over its amorphous counterpart, including:
- High concentration and high purity: In the crystalline form, the protein molecules exist in the most concentrated form and retain an active conformation;
- Possibility of controlled release: The dissolution rate in aqueous solution is low relative to the amorphous material. It may be considered as a carrier-free controlled release form;
- Increased stability: The physical and chemical stabilities of crystalline material may be greater compared to liquid solutions or the amorphous state due to higher purity, more compact molecular arrangement, and restricted molecular motion.
Creative Biostructure provides crystallization screening using a series of crystallization techniques from vapor diffusion to microbatch screening. Initial screening is conducted on a small scale using different vapor diffusion techniques (sitting drop, hanging drop, sandwich drop, and capillary) and batch crystallization techniques (slow evaporation, fast evaporation, and anti-solvent precipitation). The crystallization conditions will be further optimized and scaled up. The batch crystallization process can be applied to both small and large scale purification.
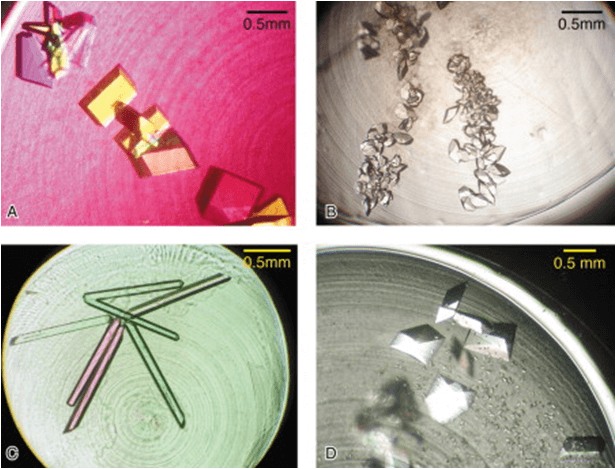 Figure 1. Collage of Crystallization Results from Small-Scale Batch Experiments
Figure 1. Collage of Crystallization Results from Small-Scale Batch Experiments
Creative Biostructure uses various leading experimental packages for advanced crystals screening and production. We are pleased to inform you the specifications for our microbatch crystallization service as follows:
- Non-disclosure agreements can be signed.
- Crystallization screening of at least 1,000 conditions by vapor diffusion method including temperatures of 4°C and 20°C.
- Optimization of crystallization condition by batch method.
- Large-scale crystal production by batch method.
- Delivery of large-scale peptide crystals encapsulated in some conical tubes (15 mL volumes) or plates.
- Each report should be submitted within 10 days from the end of each step.
Please feel free to contact us for a detailed quote.
Ordering Process
Reference
- IvanRayment. (2002) Small-Scale Batch Crystallization of Proteins Revisited: An Underutilized Way to Grow Large Protein Crystals. Structure. Pages 147-151.
