Liposomes for Nutrients
Although the utilization of liposomes is mainly directed at target drug delivery, its versatility now has been increasingly applied in dietary and nutritional supplements. Creative Biostructure offers manufacturing and analysis service to pioneer the benefit of liposomes used as nutritional supplements carriers.
Why use liposomes in nutrients?
Employment of liposome in nutrient supplements is due to the low absorption and bioavailability rate of oral dietary. The stability of oral drugs is greatly affected by the low pH of stomach, the presence of bile salts and the presence of lipases. Therefore oral liposome delivery system has been developed to bypass the gastric system and directly deliver to the target cells and tissues. With its enhanced bioavailability, nutrient solubility and stability, liposomal supplements effects are significantly increased due to increased absorption and minimal side effects. Over 50 products and product combinations have been formulated using this novel liposomal delivery system. Glutathione and vitamin C are two successful examples.
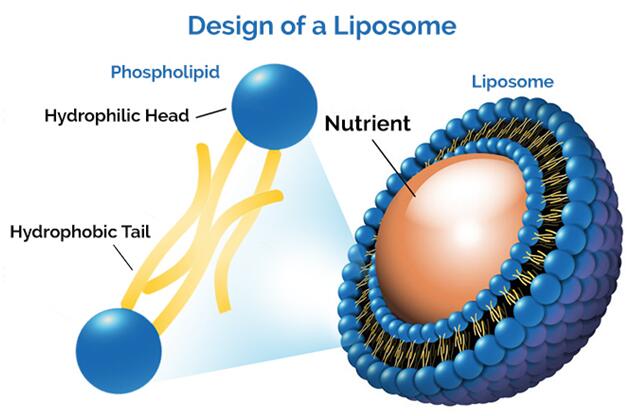 Figure 1. Basic design of liposome.
Figure 1. Basic design of liposome.
Noticing the rapid growth of liposomes use in the nutritional industry, we offer the following services to support your research.
- Liposomal formulation design for supplements: Vitamin C; Coenzyme Q10, Glucosamine sulfate and so on.
- Provide services for supplements encapsulation.
- Characterization of liposomal supplements.
Creative Biostructure has reproducible and high-quality production techniques. Creative Biostructure is able to produce high-quality products with characterized size, stability and efficiency. Please contact us for more information.
Ordering process
References
- B.C. Keller. (2001) Liposomes in nutrition. Trends in Food Science & Technology. 12: 25–31.
- Christopher W. Shad. (2016) Liposomes as advanced delivery systems for nutraceuticals. integrative medicine. 15:33-36.
- Yechezkel Barenholz. (2001) Liposome application: problems and prospects. Journal of Controlled Release. 6: 66-77.
