In Vivo FRET Assay
Creative Biostructure can efficiently and accurately study the interactions and dynamic changes between target proteins through in vivo FRET assay services, providing strong support for biological research and drug development.
What Is In Vivo FRET Assay?
Fluorescence resonance energy transfer (FRET) technology hinges on the transfer of energy between two fluorescent molecules (a donor and an acceptor). When the donor molecule absorbs energy and becomes excited, it can transfer this energy to a nearby acceptor molecule if their emission and excitation spectra overlap and they are within 1-10 nanometers of each other. This energy transfer is non-radiative and results in a decrease in donor fluorescence intensity and an increase in acceptor fluorescence intensity, which can be measured.
The in vivo FRET assay is an advanced technique used to investigate the interactions and structural dynamics of biological molecules within living organisms or cells. This method allows researchers to study the dynamics, interactions, and structures of biomolecules in their native environment, providing real-time and non-invasive measurements.
In an in vivo FRET assay, fluorescent proteins or dyes are genetically or chemically introduced into the living cells. The donor and acceptor molecules are typically tagged to specific proteins or other biomolecules of interest. Under appropriate conditions, the interaction between tagged molecules can be detected and quantified in real-time by measuring the changes in fluorescence intensity.
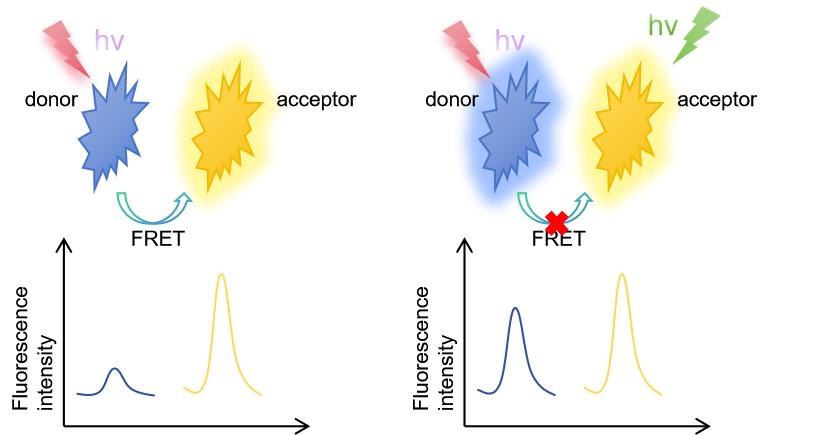 Figure 1. FRET process. (Hochreiter B, et al., 2019).
Figure 1. FRET process. (Hochreiter B, et al., 2019).
Why Perform In Vivo FRET Assay?
- Understanding Molecular Interactions In situ: In vivo FRET provides real-time data on molecular interactions within the natural, complex environment of a living cell. Dynamic changes can be observed in interactions as they occur, offering a more accurate representation than in vitro studies.
- Real-Time Monitoring: In vivo FRET can monitor the interactions and dynamic changes between biological molecules in real time, providing you with timely experimental data.
- High Sensitivity and Specificity: FRET assays are highly sensitive and can detect interactions between molecules even at very low concentrations. By tagging specific molecules with donor and acceptor fluorophores, you can study the precise interactions of interest without interference from other cellular components.
- Non-Invasive: The non-invasive nature of fluorescence detection ensures that cellular processes are not significantly perturbed during observation, preserving the physiological conditions of the cell or organism.
- Versatile Applications: Elucidating cellular signaling pathways by monitoring the interactions between signaling proteins. Studying how proteins fold and change conformation within the cellular environment. Testing the effects of potential therapeutic compounds on molecular interactions within living cells.
Our In Vivo FRET Assay Services
Creative Biostructure provides professional FRET assay services in E. coli. We use advanced gene synthesis and vector construction technology to fuse target proteins with different fluorescent tags, achieving visual observation of interactions between target proteins under a microscope through the transformation and cultivation of E. coli. Here is an overview of our in vivo FRET assay workflow:
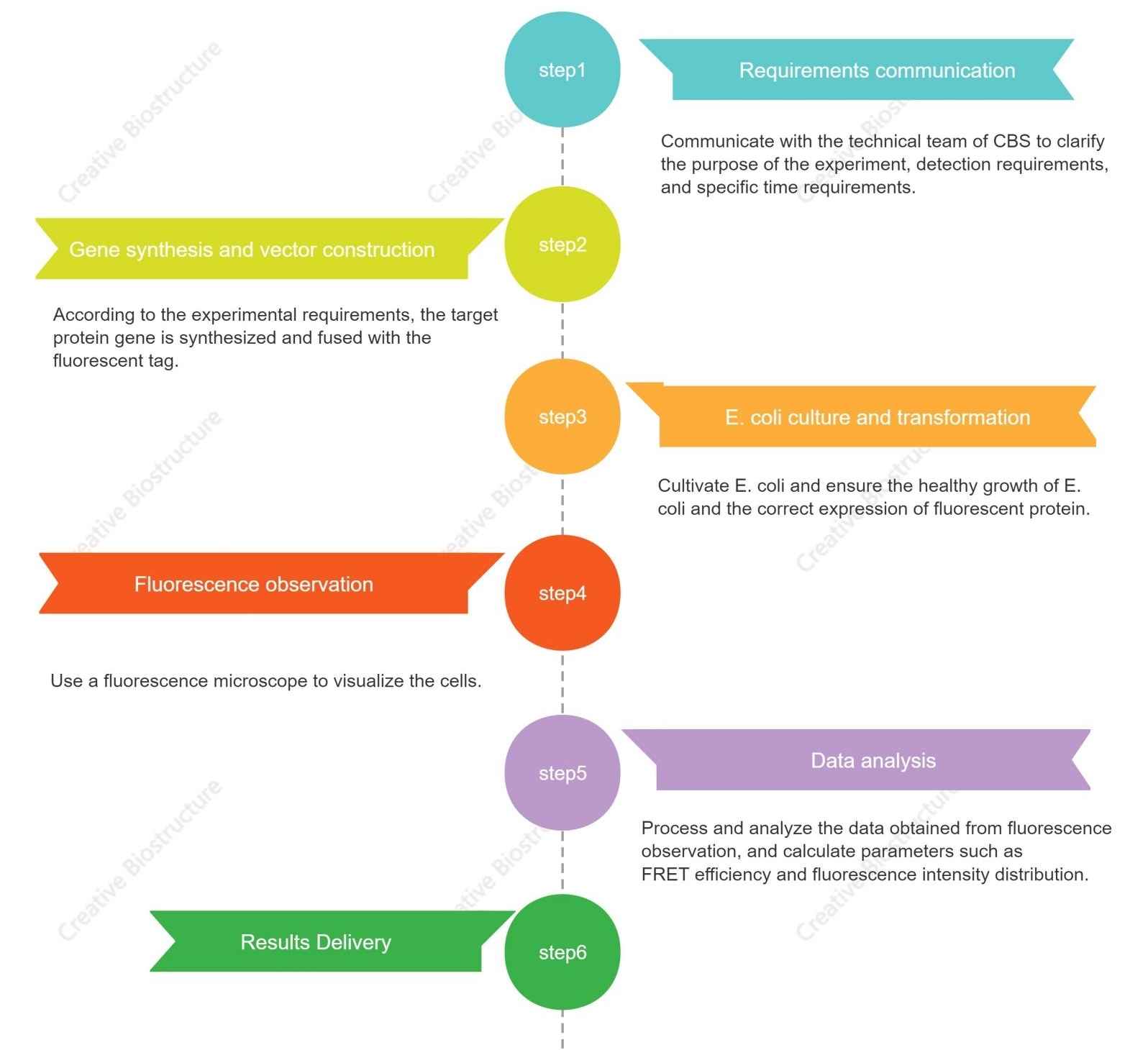 Figure 2. Our in vivo FRET technology and project workflow. (Creative Biostructure)
Figure 2. Our in vivo FRET technology and project workflow. (Creative Biostructure)
1. Requirements Communication: The initial phase involves a thorough communication with our technical team to clarify the objectives of the experiment, the specific detection requirements, and any time constraints.
2. Gene Synthesis and Vector Construction: Based on the experimental requirements, we synthesize the target protein gene and fuse it with the appropriate fluorescent tags.
3. E. coli Culture and Transformation: We cultivate E. coli, ensuring its healthy growth and the correct expression of the fluorescent protein.
4. Fluorescence Observation: Utilizing a fluorescence microscope, we visualize the cells to observe the fluorescence emitted by the tagged proteins. This observation is a direct method of assessing the interaction between the target proteins.
5. Data Analysis: Post fluorescence observation, we process and analyze the data obtained. This includes calculating parameters such as FRET efficiency and the distribution of fluorescence intensity. Our team of experts ensures that the data is interpreted accurately to provide meaningful insights into the molecular interactions.
6. Results Delivery: The final step involves the delivery of results, where we present the findings in a comprehensive report. This report outlines the outcomes of the FRET assay, providing a clear understanding of the interactions observed in the experiment.
Creative Biostructure efficiently and accurately studies the interactions and dynamic changes between target proteins using in vivo FRET detection. If you are interested, please contact us for a detailed quote.
Key Advantages of Our Services
- Precision: Our services are backed by advanced technology, ensuring precise detection and analysis.
- Reliability: With a systematic workflow, we guarantee reliable results every time.
- Customization: We tailor our services to meet the specific needs of each research project.
- Expertise: Our team of skilled professionals brings extensive knowledge and experience to every project.
Frequently Asked Questions
-
How does the in vivo FRET assay distinguish between specific and non-specific interactions?
By utilizing proper controls such as non-interacting protein pairs and utilizing parallel assays with known interactors and non-interactors, we ensure the specificity of the observed interactions. Additionally, we use advanced data analysis techniques to filter out noise and non-specific signals.
-
How do you ensure the fluorophore tagging does not interfere with the natural function of the target proteins?
To ensure that fluorophore tagging does not interfere with the natural function of target proteins, we optimize the position and length of linkers between the proteins and fluorophores. Additionally, we perform functional assays to validate that the tagged proteins behave similarly to their untagged counterparts. This ensures that our observations accurately reflect the natural interactions and functions of the proteins.
-
Can you study interactions in organisms other than E. coli?
Absolutely, our in vivo FRET assay service can be adapted to study interactions in a variety of organisms beyond E. coli. We have experience working with yeast, mammalian cells, and other model organisms. Each system may require specific optimizations, but our team is well-equipped to handle these adjustments and provide you with reliable results tailored to your organism of interest.
-
What type of data analysis and reporting do you provide as part of the service?
Our deliverables include raw data, processed FRET efficiency calculations, quantitative interaction analysis, graphical representations of the results, and a detailed report interpreting the findings. We also offer follow-up consultations to discuss the results and their implications for your research or development project.
Ordering Process
References
- Hochreiter B, Kunze M, Moser B, et al. Advanced FRET normalization allows quantitative analysis of protein interactions including stoichiometries and relative affinities in living cells. Scientific Reports. 2019. 9(1): 8233.
- Yamao M, Aoki K, Yukinawa N, et al. Two new FRET imaging measures: Linearly proportional to and highly contrasting the fraction of active molecules. Plos One. 2016. 11(10): e0164254.
- Zhou Y, Wang Y, Li J, et al. In vivo FRET–FLIM reveals ER-specific increases in the ABA level upon environmental stresses. Plant Physiology. 2021. 186(3): 1545-1561.
- Carazo A, Pávek P. The use of the LanthaScreen TR-FRET CAR coactivator assay in the characterization of constitutive androstane receptor (CAR) inverse agonists. Sensors. 2015. 15(4): 9265-9276.
