Liposomal Protein Isolation
Liposomes, especially cationic liposomes, are the most widely used drug/gene delivery nanocarriers. Based on their biocompatible composition, they can be designed to carry a variety of different drugs, ensuring the local accumulation of the drug in specific tissues or even within the target cells. Upon entry into the circulatory system, liposome nanoparticles (LNPs) encounter a protein-rich environment that may adsorb to liposomes to form so-called protein crown (corona in Latin), which may control the interaction of liposomes with tissues and cells.
Once the protein corona are formed in the correct biological environment, the stability of the LNP, the nucleic acid release profile and the availability of targeting fragments for LNP recognition by cellular receptors can be readily assessed in vitro. This allows for predicting whether specific lipid components will aggregate, be released early, or fail to target specific cells due to the masking effect of protein corona. To determine which crown component is responsible for (or interferes with) the targeting behavior of LNP, Creative Biostructure can isolate and characterize the protein crown component.
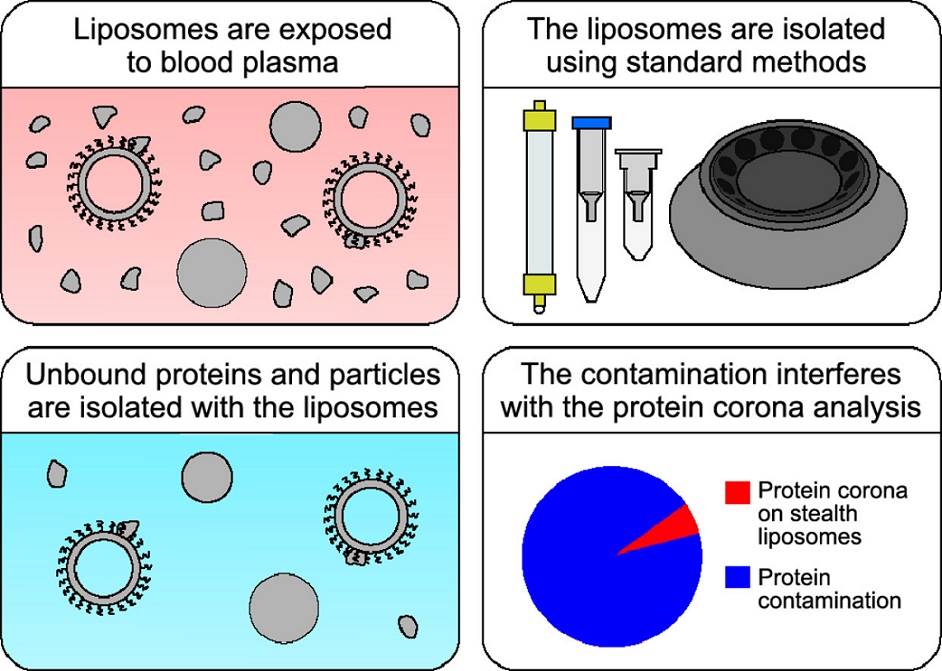 Figure 1. Isolation methods commonly used to study the liposomal protein corona suffer from contamination issues. (Kasper, K., et al., 2021)
Figure 1. Isolation methods commonly used to study the liposomal protein corona suffer from contamination issues. (Kasper, K., et al., 2021)
- Size-exclusion chromatography (SEC)
One of the most common methods for separating LNP protein corona is size- and density-based separation, an example of which is SEC, which uses gravimetric columns filled with porous resin. We can improve the SEC resolution of the column by choosing the right type of resin and increasing the column length and diameter. This technique has minimal interference with corona, and larger particles and aggregates will elute first, while loosely bound proteins of low molecular weight will elute last.
- Centrifugal separation method
Another widely used technique is the centrifugal separation method. Since the settling rate of LNPs (depending on the size and buoyancy of the LNP) is higher than that of unbound proteins, the LNP-corona complexes eventually precipitate while the unbound proteins remain in solution. Therefore, the centrifugation time and speed can be optimized according to the density of the complexes. The advantage of this method is that it is fast and only a small amount of material is available.
However, centrifugation for low-density nanoparticles such as LNP requires high gravity (ultra-centrifugation), which may lead to aggregation or disruption of LNP, which inevitably alters the corona composition. In addition, this technique often produces false positives as more proteins are separated into the same fraction as the LNPs. Therefore, several washing steps are used to remove loosely bound proteins.
In the density gradient ultracentrifugation method, LNP-protein crown complexes are first loaded into a gradient density medium and then ultracentrifuged. We can collect fractions of different densities and analyze them. This method is not as aggressive as the traditional centrifugal separation method because the particles will remain in suspension and may remain intact.
- Magnetic separator method
We load the LNP-corona complex of magnetic LNPs into a magnetic separator, wash the loosely bound proteins, and then elute the LNP-corona complex, which can be separated from the unbound proteins. This technique is the only one that can easily distinguish between particles and lipoproteins because particles and lipoproteins are not magnetic and therefore are not retained in the column.
In the serum environment, liposomes inevitably adsorb biomolecules such as proteins and form protein crown structures. Protein corona can also alter the integrity of LNPs, leading to premature release of the particles and affecting drug biodistribution and circulation times. Creative Biostructure offers solutions for the separation of LNP-protein crown complexes.
After the incubation separation of NPs and their crowns, we can usually provide liquid chromatography-mass spectrometry (LC-MS) analysis of the protein content, which has become the gold standard for qualitative and quantitative identification of the individual proteins forming the coronas. Other available techniques can identify the macroscopic effects of corona on nanoparticles, such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), electrophoretic light scattering (ELS, ζ-potential), and (low temperature) transmission electron microscopy (TEM), or elucidate more subtle protein changes, such as nuclear magnetic resonance spectroscopy (NMR), fluorescence correlation spectroscopy (FCS), ultracentrifugation, surface plasmon resonance (SPR), etc. Feel free to contact us to learn more about available techniques.
Ordering Process
Reference
- Kristensen K., et al. Isolation methods commonly used to study the liposomal protein corona suffer from contamination issues. Acta Biomaterialia. 2021, 130: 460-472.
