Detergent Screening for Membrane Protein Purification
Membrane proteins consist of one or more hydrophobic regions that are buried in the membrane as well as hydrophilic regions that contain charged or polar residues that are exposed to water. For crystallization, membrane proteins are usually solubilized from the membranes using a detergent and then purified in the presence of a detergent. The membrane proteins thus prepared are water-soluble protein–detergent complexes in which the membrane-anchored hydrophobic portions are covered with amphiphilic detergent molecules.
Many membrane proteins have been crystallized, however, the crystallization of membrane proteins is still a difficult task and the quality of the crystals obtained has often been insufficient for X-ray diffraction studies. One of the obstacles in the crystallization of membrane proteins is the requirement of detergent, which is used to extract the protein from the lipid bilayer to form a protein-detergent complex (PDC). Understanding the variables associated with detergent phase behavior and crystallogenesis enables their rational modulation.
Creative Biostructure has generated chemically diverse phase boundary data utilizing dye-based phase partitioning and magic kits of detergents commonly used to crystallize membrane proteins. The resulting data have been used to guide the formulation of a cocktail format of membrane protein crystallization screen. The experimentally derived phase boundary data and the membrane screen are now available through the high-throughput crystallization facility. The selection of the right detergent is crucial for the effective purification of a membrane protein. Factors to bear in mind are: (1) the critical micelle concentration (CMC) of a detergent, or the concentration at which the detergent forms micelles and accumulates in the membrane; (2) that changing protein or salt concentration can change the aggregation behavior of a protein and the CMC of a detergent; (3) that prolonged exposure to a detergent can delipidate a protein which leads to instability; and (4) that certain detergents are suboptimal for subsequent chromatographic purification; for example, ion exchange chromatography does not work well with charged detergents.
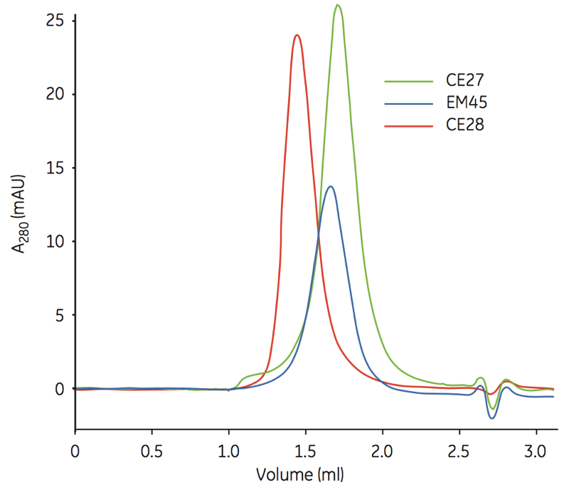 Figure 1. Quality control of concentrated protein samples.
Figure 1. Quality control of concentrated protein samples.
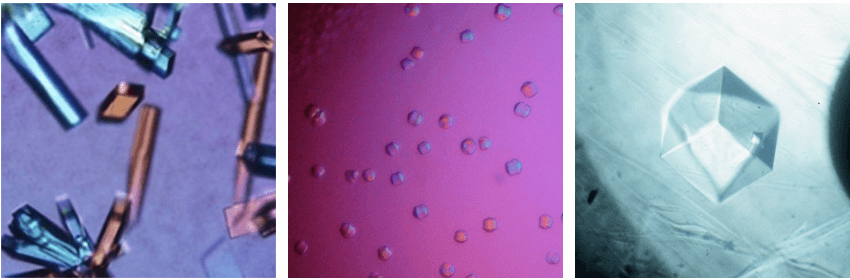 Figure 2. Integral membrane protein crystals diffracting to 4 to 14 Å maximum resolution.
Figure 2. Integral membrane protein crystals diffracting to 4 to 14 Å maximum resolution.
Creative Biostructure establishes protocols and pipelines for membrane protein expression and purification. With biophysical and biochemical characterization, we offer structural insight into disease targets for both industry and academia.
Please feel free to contact us for a detailed quote.
Ordering Process
References
- Hironari Shimizu et al. Screening of detergents for solubilization, purification and crystallization of membrane proteins: a case study on succinate: ubiquinone oxidoreductase from Escherichia coli. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Sep 1; 64(Pt 9): 858–862.
- Gellman, S.H. et al. Maltose-neopentylglycol (MNG) amphiphilesfor solubilization, stabilization and crystallization of membrane proteins. Nat Methods 7, 1003–1008 (2010).
