Application of Circular Dichroism Spectroscopy in Protein Research
At Creative Biostructure, we provide circular dichroism (CD) spectroscopy service for protein research. This potent technique empowers us to delve into the secondary and tertiary structure of proteins, offering profound insights into their folding, conformational dynamics, and interplays.
Significance of CD Spectroscopy in Protein Research
Proteins are hierarchical structures formed of amino acids linked by peptide bonds. The structure's peptide bonds, aromatic amino acid residues, and disulfide bridges are all optically active groups. The difference in proteins' secondary and tertiary structures will affect their optical activity. The above phenomenon is called circular dichroism of protein and follows certain rules in the CD spectrum.
Circular dichroism is the most widely used method for determining the secondary structure of proteins, and it is a fast, simple, and accurate method for studying protein conformation in dilute solutions. It can be measured in a solution state closer to its physiological state. Moreover, the determination method is quick and easy and sensitive to conformational changes, so it is one of the main means to study the secondary structure of proteins and has been widely used in the conformational studies of proteins.
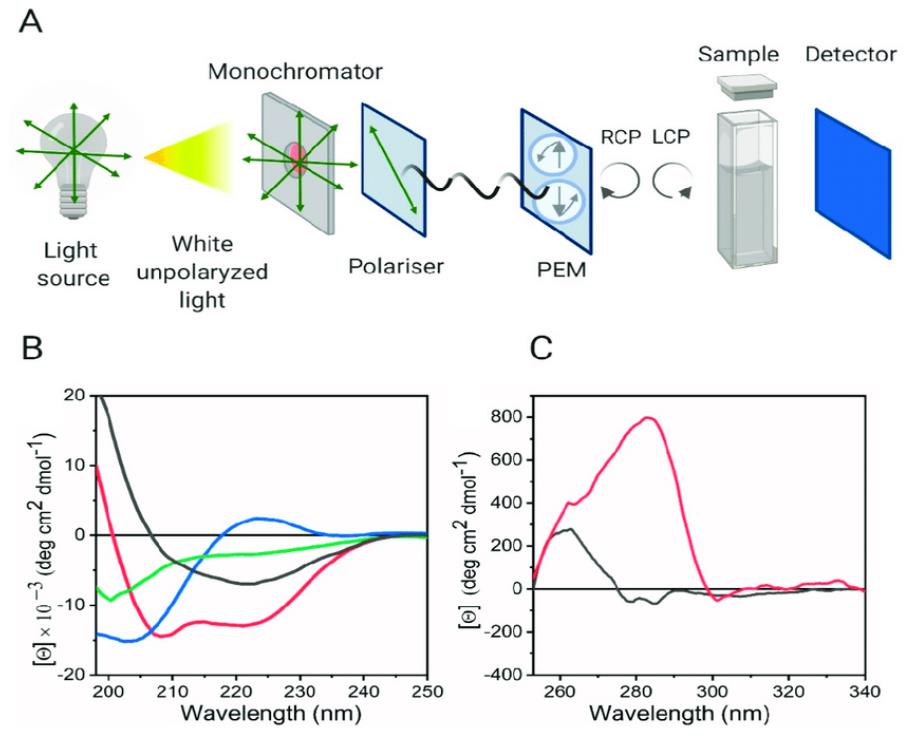 Figure 1. Circular Dichroism is a tool for studying protein secondary and tertiary structure. (Pignataro M F, et al., 2020)
Figure 1. Circular Dichroism is a tool for studying protein secondary and tertiary structure. (Pignataro M F, et al., 2020)
How to Study Protein Using CD Spectroscopy
Our service employs state-of-the-art CD spectroscopy techniques to unravel the protein's secrets. A protein solution is subjected to circularly polarized light, and the difference in absorption of left- and right-handed light is recorded. This data-rich technique provides insights into a protein's secondary structure, revealing changes due to environmental factors, ligand binding, and more. Our experts meticulously design experiments, select optimal conditions, and interpret the spectra to draw meaningful conclusions about the protein's structural dynamics.
What Can We Offer?
Creative Biostructure has rich experience in protein research, and our experienced scientists can use CD spectroscopy to help analyze the three-dimensional structure of your protein and assist your research. Our CD spectroscopy service in protein research can be used to analyze the following:
- Determining Protein Structures
Circular dichroism spectroscopy can rapidly determine the secondary structure and folding properties of proteins obtained by recombinant techniques or purified from tissue. The most widespread application of protein circular dichroism analysis is determining whether expressed purified proteins are folded or mutated, affecting their conformation or stability.
- Analysis of the Factors Affect Protein Conformation
Circular dichroism can also be used to analyze factors that affect protein conformation. By analyzing the conformation of proteins under different conditions (such as pH, temperature, ion concentration, etc.), the factors that affect protein conformation can be compared and analyzed to help protein research.
- Protein Interaction Analysis
Circular dichroism can also be used to study protein interactions and determine the interactions between proteins or between proteins and nucleic acids through changes in protein conformation. Furthermore, since CD is a quantitative technique, these changes are proportional to the number of protein-protein complexes formed and thus can also be used to estimate binding constants.
Advantages of Our Service
- Expertise: Our team consists of skilled professionals with extensive experience in both CD spectroscopy and protein research. With a deep understanding of both fields, we bring unparalleled insights to your projects.
- Customization: Recognizing the uniqueness of each protein, we customize our methods to suit your research goals, ensuring relevance and impact.
- Cutting-edge Technology: Utilizing the latest CD spectroscopy technology, we provide accurate, detailed, and current data to support your research.
- Comprehensive Analysis: Our comprehensive analysis includes advanced spectral deconvolution, quantitative secondary structure determination, and correlation with functional changes, enabling a holistic understanding of your protein.
- Timely Delivery: We value your time. Our efficient processes ensure that you receive your results promptly, empowering you to move forward with your research without unnecessary delays.
As an expert in protein structure, Creative Biostructure has many years of experience in protein structure research, we provide circular dichroism analysis for structural characterization and intermolecular interactions of various proteins, and we promise to work closely with our customers to provide excellent services. Contact us today to discuss how we can advance your research and contribute to groundbreaking insights in the world of structural biology.
Ordering Process
References
- Hoffmann S V, Fano M, van de Weert M. Circular dichroism spectroscopy for structural characterization of proteins. Analytical Techniques in the Pharmaceutical Sciences. 2016: 223-251.
- Gekko, K. Carbohydrate Circular Dichroism. Encyclopedia of Biophysics. Springer, Berlin, Heidelberg. 2018, 214-216.
- Pignataro M F, Herrera M G, Dodero V I. Evaluation of peptide/protein self-assembly and aggregation by spectroscopic methods. Molecules. 2020, 25(20): 4854.
