Epitope Mapping by NMR
Creative Biostructure specializes in identifying conformational epitopes using structural biology technologies. The star strategies include X-ray crystallization, Nuclear Magnetic Resonance (NMR) and 3D-Electron Microscope (3D-EM) to obtain information about the structure of peptides, proteins, and their complexes. Among which, NMR is a gold standard technique for determining the boundaries of peptide epitopes recognized by antibodies. If you wish to screen against multiple protein targets, this can be done simultaneously for rapid analysis.
NMR spectroscopy is emerging as the technique of choice to map allosteric phenomena at the atomic level, unveiling mechanisms underlying allostery. Heteronuclear correlation experiments provide NMR amide “fingerprints” of proteins and protein complexes. Choice of the specific method depends upon the dissociation constant, especially the ligand off-rate. Epitope mapping by NMR is based on the differences in mobility between the amino acid residues of a peptide antigen that interact tightly with the antibody and residues outside the epitope that do not interact with the antibody. The interacting peptide residues become considerably immobilized upon binding. Their mobility will resemble that of the antibody's residues.
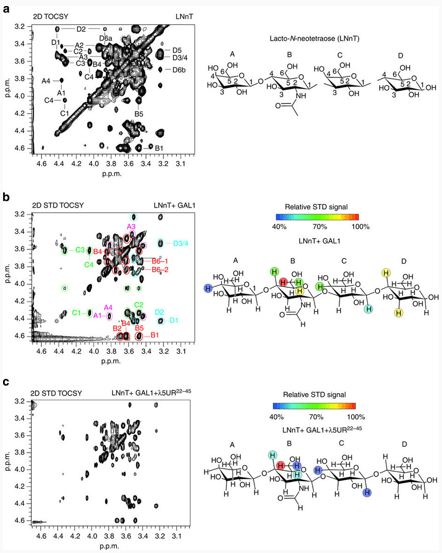 Figure 1. Mapping the LNnT-binding epitope using 2D STD-NMR spectroscopy.
Figure 1. Mapping the LNnT-binding epitope using 2D STD-NMR spectroscopy.
NMR epitope mapping provides more detailed information than mutagenesis or peptide mapping and can be much more rapid than X-ray crystallography.
Creative Biostructure can undertake:
- Screening-hit validation – assessment of binding behavior; footprint of binding site
- Primary screening to discover hits – from conventional libraries to fragments
- 3D structures of proteins, protein-ligand complexes, peptides, macrocycles
- Assessment of protein physical and folding status for checking batches, new constructs, or modified forms
- Application of advanced NMR methods on small molecules – identification of primary structure or stereochemistry
- Consulting – advice on protein-protein interaction targets in drug discovery, fragment screening, screening library design, and peptide design
NMR can determine structures and epitopes of:
- Proteins
- Protein-ligand complexes
- Peptides
- Macrocycles
NMR structures of peptides allow pharmacophore models to be derived for virtual screening to find non-peptidic analogs.
Besides NMR, Creative Biostructure offers a field of structural biology for mapping protein epitopes, such as Epitope Mapping by crystallography and 3D-EM. Applications include epitope mapping, antibody engineering (affinity maturation and humanization), and protein engineering.
Please feel free to contact us for a detailed quote.
Ordering Process
References:
- Rosen O1, Anglister J., Epitope mapping of antibody-antigen complexes by nuclear magnetic resonance spectroscopy. Methods Mol Biol. 2009;524:37-57. doi: 10.1007/978-1-59745-450-6_3.
- M. Bardelli, et al. Epitope mapping by solution NMR spectroscopy. Journal of Molecular Recognition. 27 Feb 2015. DOI: 10.1002/jmr.2454.
- Jeremy Bonzi, et al. Pre-B cell receptor binding to galectin-1 modifies galectin-1/carbohydrate affinity to modulate specific galectin-1/glycan lattice interactions. Nature Communications. 24 Feb 2015. doi:10.1038/ncomms7194
