Bacterial Extracellular Vesicle Services
To harness the full potential of bacterial extracellular vesicles (BEVs) for advanced therapeutic and diagnostic applications, it is crucial to have reliable methods for their extraction, purification, characterization, and analysis. Creative Biostructure offers comprehensive BEV services to meet these needs, enabling precise and efficient utilization of BEVs in cutting-edge biomedical research.
What Are Bacterial Extracellular Vesicles (BEVs)?
BEVs are nanosized vesicles derived from bacteria that play a crucial role in intercellular communication by transferring bioactive molecules to recipient cells. They are characterized by their lipid bilayer composition and can carry a variety of cargo, including proteins, nucleic acids, lipids, and metabolites. BEVs are comparable to liposomes and have emerged as a novel platform for drug delivery due to their biocompatibility and ability to encapsulate and transport various bioactive molecules.
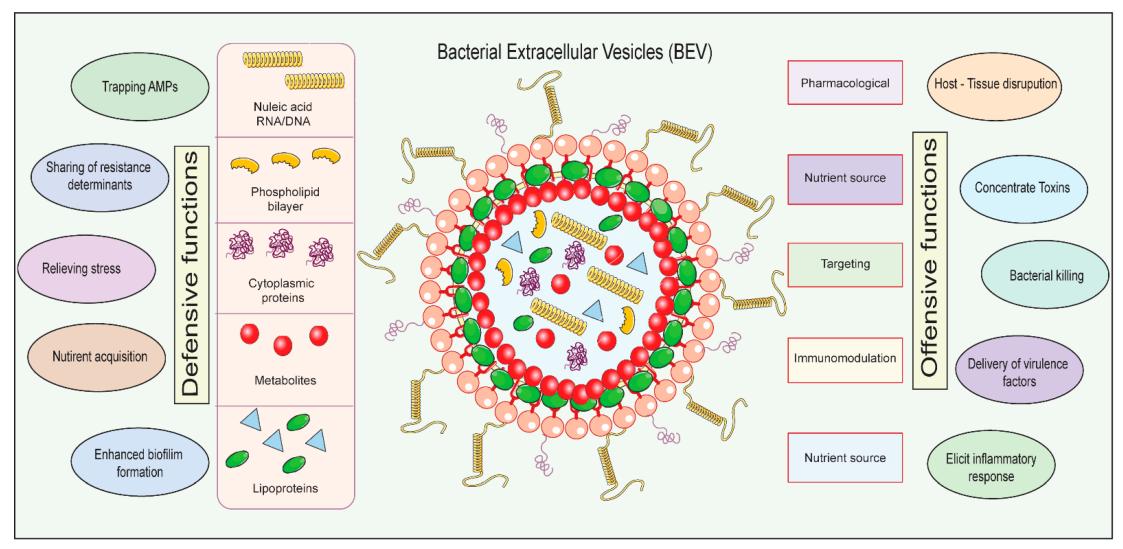 Figure 1. The structure and application of BEVs. (Iyaswamy
A, et al., 2023).
Figure 1. The structure and application of BEVs. (Iyaswamy
A, et al., 2023).
Advantages Over Traditional Drug Delivery Systems
- Cost-Effective Production: Bacteria's rapid proliferation makes BEV production more efficient and less costly compared to traditional drug delivery systems.
- Precision and Customization: Modern gene editing tools allow for the precise manipulation of BEV composition and functionality. This facilitates the development of targeted treatments tailored to specific diseases.
- High-Density Culture Technology: Advances in culture technology enable mass production of BEVs. This industrial scalability ensures a steady supply of vesicles for clinical applications.
- Synthetic Biology Applications: Synthetic biology opens new avenues for creating multifunctional and customized BEVs. By designing and modifying bacteria, scientists can generate BEVs with specific properties aimed at particular diseases. These engineered BEVs can transport therapeutic drugs, immunomodulatory molecules, or diagnostic markers with high precision, improving the effectiveness of disease treatment.
Bacterial Extracellular Vesicle Services at Creative Biostructure
Creative Biostructure is dedicated to advancing BEV research and applications by offering a suite of specialized services. Our comprehensive approach ensures that each aspect of BEV study, from extraction to microbiomics analysis, is conducted with precision and expertise.
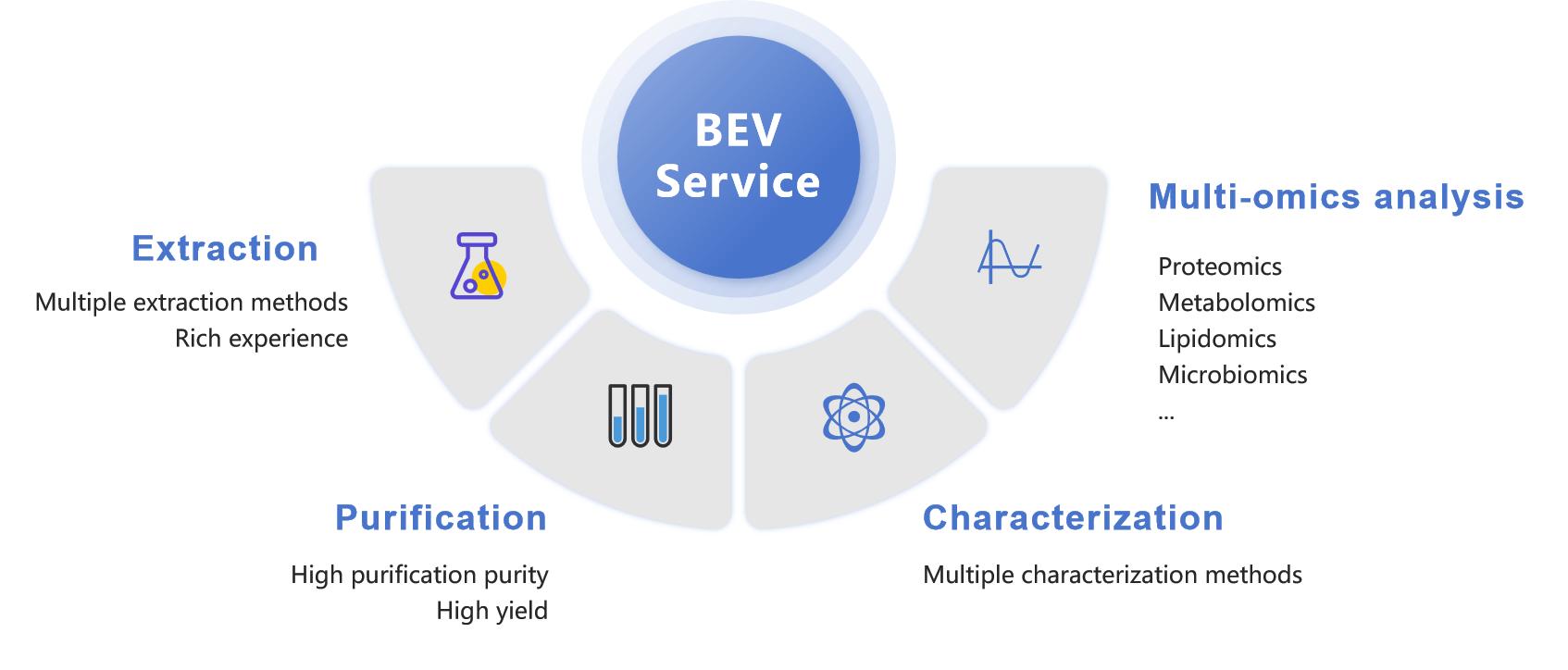 Figure 2. Various BEV services. (Creative Biostructure)
Figure 2. Various BEV services. (Creative Biostructure)
Bacterial Extracellular Vesicle Extraction
Our extraction services employ a range of state-of-the-art techniques to isolate BEVs effectively. Utilizing methods such as PEG precipitation, which aids in the concentration of vesicles, ultracentrifugation for high-speed separation, magnetic bead extraction for targeted isolation, and density gradient centrifugation to purify based on density differences, we ensure the recovery of high-quality BEVs. These methods are meticulously chosen to suit various research requirements and sample types, providing a robust foundation for downstream applications.
Bacterial Extracellular Vesicle Purification
Purification is a critical step following extraction, and we achieve this through a meticulous process. Initially, low-speed centrifugation removes bacteria and debris from the fermentation broth. This is followed by sterile filtration to eliminate any residual bacteria and proteins unrelated to BEVs. Our purification protocols are designed to obtain BEVs of the highest purity, ensuring that subsequent analyses and applications are not confounded by contaminants.
Bacterial Extracellular Vesicle Characterization
Characterization of BEVs is essential to understand their properties and behavior. We offer a suite of characterization services, including transmission electron microscopy (TEM) imaging for morphological assessment, nanoparticle tracking analysis (NTA) for size distribution and concentration measurements, Western Blot for protein profiling, and flow cytometry for surface marker analysis. Additionally, we provide exosome quantitative analysis to determine the yield and purity of the BEV preparation, ensuring that our clients have a thorough understanding of their samples.
Bacterial Extracellular Vesicle Microbiomics Analysis
Recognizing BEVs as crucial communicators in the intricate dialogue between intestinal microorganisms and host cells, our microbiomics analysis service is pivotal. We have established an advanced platform that leverages cutting-edge sequencing technologies and bioinformatics tools to dissect the microbial composition associated with BEVs. This analysis is vital for understanding the role of BEVs in gut microbiome-host interactions and their potential implications in health and disease. Our microbiomics analysis provides insights into the functional potential of the microbiota encapsulated within BEVs, paving the way for targeted therapeutic strategies.
With our extensive project experience and expertise in the field of exosomes, Creative Biostructure is committed to providing customers with accurate and detailed data support and meeting diverse needs through our top-tier BEV services. If you are interested in our offerings, please contact us for a detailed quote.
Technology Platforms
Key Advantages of Our BEV Services
- Customized Service Approach: Each step of our service is customizable to meet the unique requirements of your project, whether it's the extraction method, purification stringency, or characterization scope, ensuring that your BEV research is supported with the highest flexibility and precision.
- Expertise in BEV Applications: Our team's deep expertise in BEV applications, from basic research to clinical translation, means we can provide informed guidance and support throughout your project, helping you navigate the complexities of BEV research and development.
- Quality Assurance and Precision: We are committed to maintaining the highest standards of quality and precision in all our services, ensuring that every BEV sample is processed with the utmost care and accuracy.
- Supportive and Knowledgeable Team: Our team of scientists and technical experts is always ready to provide support and advice, ensuring that your BEV research is not only technically successful but also scientifically meaningful.
Resources
Frequently Asked Questions
-
Can the services be customized to accommodate specific research objectives?
Yes, customization is at the core of our service offerings. We understand that each research project has unique requirements and can tailor our extraction, purification, and analysis methods to focus on specific BEV subsets or cargo molecules. Our team works closely with you to understand your goals and customize the service accordingly.
-
How do you ensure that the extraction and purification processes do not compromise the integrity and biological activity of the BEVs?
We employ gentle and optimized protocols for BEV extraction and purification that are designed to preserve the integrity and biological activity of the vesicles. We utilize state-of-the-art techniques that minimize stress on the BEVs, ensuring that they retain their functional properties for subsequent applications.
-
What is the typical turnaround time for the complete BEV service, from extraction to final analysis?
Our turnaround times are competitive and designed to meet the needs of our clients without compromising quality. Depending on the complexity of the service, we typically provide a timeline estimate upfront. In cases of urgent need, we can work with you to expedite the process while maintaining the highest standards of service.
Ordering Process
References
- Liu H, Zhang Q, Wang S, et al. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioactive Materials. 2022. 14: 169-181.
- Chen J, Zhang H, Wang S, et al. Inhibitors of bacterial extracellular vesicles. Frontiers in Microbiology. 2022. 13: 835058.
- Iyaswamy A, Lu K, Guan X J, et al. Impact and advances in the role of bacterial extracellular vesicles in neurodegenerative disease and its therapeutics. Biomedicines. 2023. 11(7): 2056.
 Exosome Solutions for Your Proceeding Research
Exosome Solutions for Your Proceeding Research
 Exosomes Products
Exosomes Products Exosome Characterization
Exosome Characterization Custom Nano-Flow Cytometry Analysis
Custom Nano-Flow Cytometry Analysis