X-ray diffraction (XRD) is a cornerstone analytical technique for studying the atomic and molecular arrangements within crystalline materials. Within the XRD family, single crystal X-ray diffraction (SCXRD) and powder X-ray diffraction (PXRD) represent two main approaches, each with different capabilities and applications. Understanding the differences between these techniques is critical to selecting the optimal method for structural analysis in diverse fields such as research, pharmaceuticals, materials science, and industry. This article examines the principles, strengths, weaknesses, and applications of SCXRD and PXRD to help determine the most appropriate approach for specific analytical needs.
What is X-ray Diffraction (XRD)?
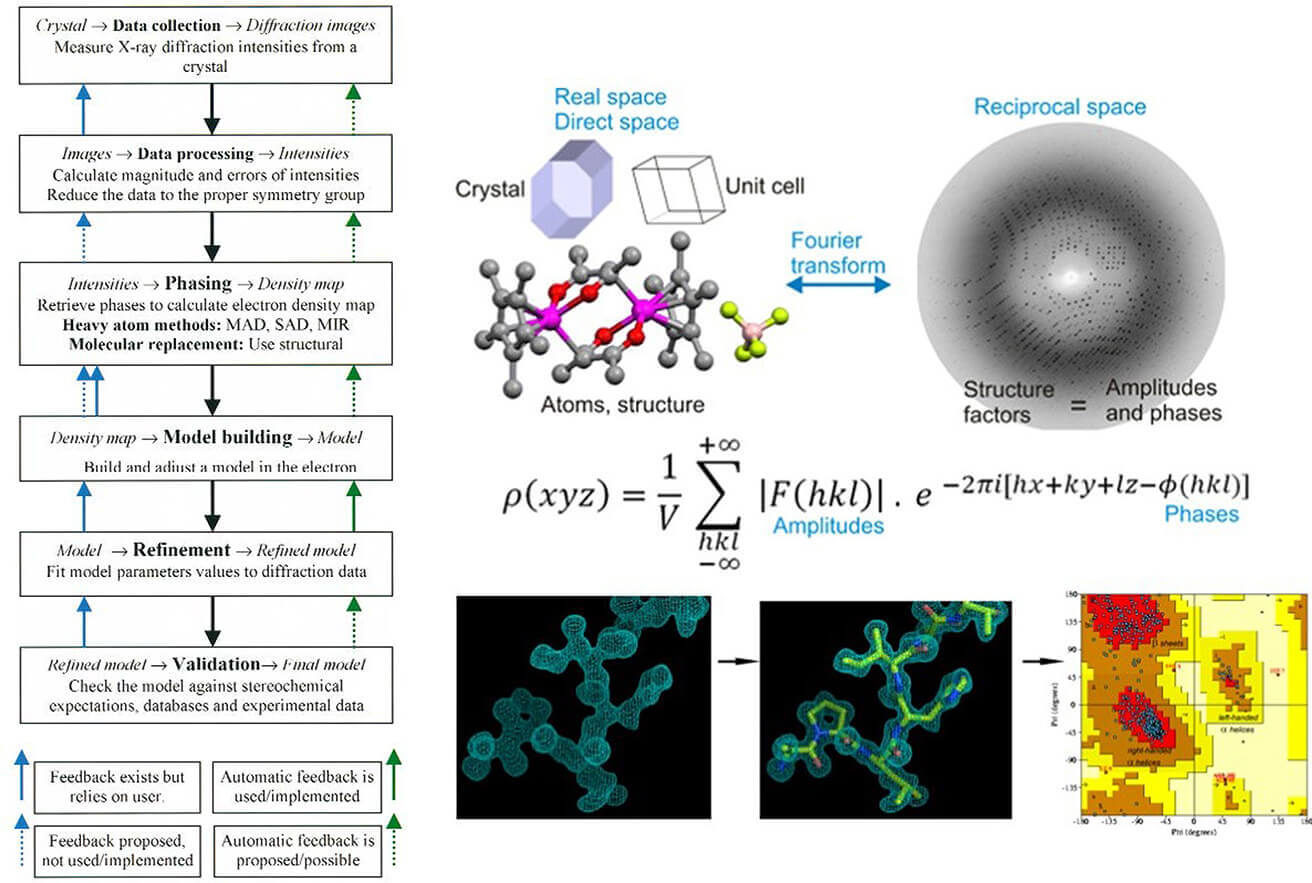
X-ray diffraction is a powerful tool for studying the atomic architecture of crystalline materials. When X-rays strike a crystal lattice, they diffract along specific trajectories, creating a pattern that provides detailed structural information upon analysis. The fundamental principle governing this phenomenon is Bragg's Law, which articulates the conditions for constructive interference of X-rays scattered by the atomic planes of the crystal. This diffraction pattern acts as a unique identifier for the material, allowing researchers to determine unit cell dimensions, atomic coordinates, and overall structural composition.
Overview of Single Crystal X-ray Diffraction (SCXRD)
Fundamental Principles of SCXRD
SCXRD is an analytical technique known for determining the atomic structure of crystalline materials with exceptional precision. It works by directing a focused, monochromatic X-ray beam onto a single crystal and meticulously measuring the diffracted rays. The resulting diffraction pattern, governed by Bragg's Law, provides comprehensive data about the crystal lattice, including atomic positions, bond lengths, and angles. Unlike PXRD, which examines an ensemble of randomly oriented crystallites, SCXRD focuses on a well-formed crystal, establishing it as an essential tool for detailed structural elucidation.
To understand SCXRD in detail, check out our article: What Is Single-Crystal X-ray Diffraction (XRD) and How Does It Work?
SCXRD Sample Requirements and Preparation
The effectiveness of SCXRD is highly dependent on crystal quality. A well-defined, defect-minimized crystal is essential for obtaining interpretable diffraction data. Ideally, the crystal should have adequate size (typically ≥ 0.1 mm in one dimension) and smooth facets. Suitable crystals are grown using methods such as slow evaporation, vapor diffusion, or melt crystallization. Once obtained, the crystal is mounted on a goniometer, often using a fiber optic or a loop of cryoprotective oil. Cryogenic cooling is often used to reduce thermal motion and mitigate radiation damage, thereby improving data accuracy.
SCXRD Data Collection Process
SCXRD data acquisition involves systematically rotating the crystal within the X-ray beam while recording the intensities of the diffracted X-rays at numerous orientations. This rotation produces a series of discrete diffraction spots, each corresponding to specific atomic planes. A high-resolution detector captures these spots, and the collected data set facilitates the reconstruction of the three-dimensional electron density map of the material. Unlike the diffraction rings produced by random particle orientation in PXRD, SCXRD produces discrete spots that provide a highly detailed view of atomic arrangements, often achieving sub-angstrom resolution.
Advantages and Limitations of SCXRD
SCXRD has significant advantages, particularly its unparalleled resolution, ability to determine precise atomic positions, and non-destructive nature. It is particularly valuable in pharmaceutical research for the analysis of drug polymorphism and in materials science for the characterization of novel functional compounds. However, SCXRD has limitations. The need for high quality single crystals is challenging, as certain substances resist the formation of suitable crystals. In addition, data collection and subsequent structure refinement require sophisticated instrumentation and considerable expertise, making the technique less accessible than PXRD. Despite these hurdles, SCXRD remains the benchmark for crystallographic analysis because of its ability to reveal atomic-level structural intricacies.
Overview of Powder X-ray Diffraction (PXRD)
Fundamental Principles of PXRD
PXRD is a widely used technique for analyzing the crystallographic structure of materials presented as powders. Unlike SCXRD, which requires a single crystal, PXRD analyzes a large collection of randomly oriented microcrystals (crystallites). The interaction of X-rays with this powder produces a diffraction pattern characterized by concentric rings rather than discrete spots. The resulting plot of intensity versus diffraction angle (2θ), interpreted by Bragg's Law, provides essential structural information, allowing the determination of phase composition, crystallinity and lattice parameters. Its efficiency and ease of sample preparation make PXRD widely used in materials science, pharmaceuticals, and geology. For a deeper understanding of PXRD, visit our dedicated resource: Overview of Powder X-ray Diffraction (PXRD).
PXRD Sample Preparation and Measurement
Compared to SCXRD, PXRD requires significantly less sample preparation, making it suitable for routine analysis. The material is typically ground into a fine powder to ensure random orientation of the crystallites to provide a representative diffraction pattern. This powder is then packed into a sample holder, often compacted for uniformity, and exposed to monochromatic X-rays. A detector records the intensity of the scattered X-rays as a function of the 2θ angle. The output diffractogram serves as a unique "fingerprint" for each crystalline material, facilitating phase identification by comparison to reference databases. Careful sample preparation is essential as particle size, preferred orientation and sample thickness can all affect analytical accuracy.
PXRD Data Interpretation and Structural Analysis
The PXRD diffractogram shows peaks corresponding to different crystallographic planes. By analyzing the positions, intensities, and shapes (widths) of these peaks, researchers can extract valuable data about the sample's crystal structure(s), phase purity, and degree of crystallinity. Diffraction data are often compared with standard patterns from databases such as the International Center for Diffraction Data (ICDD) for compound identification. In addition, sophisticated computational techniques, particularly Rietveld refinement, allow precise determination of unit cell parameters and, in favorable cases, atomic coordinates. PXRD is particularly effective for materials that are difficult to grow as large single crystals, providing a practical route to structural characterization. If you're interested in the role of PXRD in pharmaceutical research, check out our article: X-ray Powder Diffraction in Drug Polymorph Analysis.
Advantages and Limitations of PXRD
PXRD offers numerous advantages that solidify its role in crystallographic studies. A key strength is its rapid analysis capability, often providing data within minutes, making it ideal for high throughput screening. PXRD easily handles multiphase mixtures, proving invaluable for the analysis of complex samples such as pharmaceutical formulations, minerals and composites. It is also non-destructive. However, PXRD has limitations. Its resolution is inherently lower than SCXRD, and it typically provides phase identification and unit cell data rather than high-precision atomic positions for unknown structures. The analysis often relies on existing reference patterns, potentially limiting its utility for entirely new materials. Despite these limitations, PXRD remains an important technique for qualitative and quantitative phase analysis.
Key Differences Between Single Crystal XRD and Powder XRD
SCXRD and PXRD are two major crystallographic techniques used to analyze the structure of crystalline materials. While both are based on the principles of X-ray diffraction, they differ significantly in terms of sample requirements, data output, applications, and structural resolution. Below we break down the key differences between these two methods to highlight their respective advantages and limitations.
1. Sample Requirements and Preparation
SCXRD: This technique requires a high quality single crystal with well defined faces and minimal defects. The sample must be sufficiently large (typically >50 μm) and well ordered to allow the collection of distinct diffraction spots. Preparing a suitable single crystal often requires optimized crystallization conditions and extensive trial and error, making SCXRD less practical for materials that do not readily form large crystals.
PXRD: In contrast, PXRD works with a finely powdered sample, eliminating the need for large single crystals. The powder consists of numerous randomly oriented microcrystals, ensuring that all possible crystal orientations contribute to the diffraction pattern. Sample preparation is minimal, usually involving simple grinding and packing, making PXRD more accessible and faster than SCXRD.
2. Diffraction Pattern and Data Collection
SCXRD: The diffraction pattern of a single crystal consists of discrete, well-defined spots, each corresponding to a specific set of atomic levels within the crystal lattice. By systematically rotating the crystal and collecting diffraction intensities at different angles, a three-dimensional data set is created that allows precise determination of the atomic structure.
PXRD: Instead of individual diffraction spots, PXRD produces a continuous diffraction pattern in the form of concentric rings. The final data is presented as an intensity vs. diffraction angle (2θ) plot where peak positions correspond to specific lattice spacings. Since all crystal orientations are sampled simultaneously, PXRD does not require complex crystal rotation, but provides less detailed structural information.
3. Structural Information and Resolution
SCXRD: One of the greatest advantages of SCXRD is its high resolution, often at the atomic level. The technique allows the direct determination of bond lengths, angles, electron density distributions, and molecular conformations, making it the gold standard for solving complex crystal structures, including proteins, small organic molecules, and inorganic materials.
PXRD: While PXRD is excellent for phase identification and crystallinity analysis, it does not provide direct atomic positions with the precision of SCXRD. Instead, PXRD data is used to refine known structures, estimate lattice parameters and analyze bulk properties. However, with advanced computational techniques such as Rietveld refinement, PXRD can still provide valuable structural insight.
4. Applications in Research and Industry
SCXRD: Due to its unparalleled structural resolution, SCXRD is widely used in molecular chemistry, drug discovery, materials science, and crystallography research. It is particularly valuable for determining the precise 3D arrangement of atoms in proteins, catalysts, pharmaceuticals, and novel materials, making it essential for understanding molecular interactions and reaction mechanisms.
PXRD: PXRD excels in phase identification, quality control, and bulk material characterization. It is widely used in pharmaceutical development to identify drug polymorphs (different crystalline forms of a drug), in materials science to study crystallinity and stress-strain behavior, and in geology to identify minerals.
5. Time Efficiency and Accessibility
SCXRD: Data collection and analysis in SCXRD can be time-consuming, often requiring several hours to days depending on crystal quality and structure complexity. In addition, not all materials readily form single crystals, making the technique less accessible for routine analysis.
PXRD: PXRD is much faster and easier to use, often providing results in minutes to hours. This makes it ideal for high-throughput screening, industrial quality control and rapid materials characterization. The technique's simplicity and minimal sample preparation requirements also contribute to its widespread adoption.
6. Limitations and Challenges
SCXRD: Despite its accuracy, SCXRD is limited by the requirement for high-quality single crystals, making it unsuitable for polycrystalline or amorphous materials. In addition, data acquisition can be complex, requiring specialized equipment and expertise.
PXRD: While versatile, PXRD suffers from peak overlap and limited structural resolution. It is not as effective as SCXRD at solving new crystal structures from scratch and relies on existing reference databases for phase identification. In addition, issues such as preferred orientation effects and peak broadening can sometimes complicate data interpretation.
How to Choose Between Single Crystal XRD and Powder XRD?
| Factor | Single Crystal XRD (SCXRD) | Powder XRD (PXRD) |
|---|---|---|
| Sample Type | Requires high-quality single crystals | Works with polycrystalline powders, bulk solids, thin films |
| Resolution | Atomic-level precision, provides bond lengths, angles, and electron density | Moderate resolution, suitable for phase identification and bulk analysis |
| Application | Best for structural elucidation in chemistry, pharmaceuticals, and materials science | Ideal for phase identification, quality control, and material characterization |
| Time & Cost | Time-intensive, costly, requires skilled expertise | Fast, cost-effective, user-friendly |
| Limitations | Requires large single crystals, not suitable for mixed or amorphous materials | Lower resolution, peak overlap issues, relies on reference patterns |
When precise atomic structures and molecular conformations are required, SCXRD is the best option, provided high quality single crystals are available. However, if the goal is phase identification, bulk characterization, or rapid analysis, PXRD is the preferred technique due to its speed, accessibility, and broad applicability.
Case Study: Comparing Powder vs. Single-Crystal X-ray Diffraction using Griseofulvin
Goal: This research tested if PXRD is as accurate as the standard SCXRD for determining the crystal structure of a small organic molecule, using griseofulvin (C17H17ClO6) as the test case.
Methods: The scientists determined the crystal structure of griseofulvin using both PXRD and SCXRD techniques and then compared the results.
Key Findings:
- Both methods produced very similar results for the crystal's basic dimensions (lattice parameters) and overall arrangement (P41 space group).
- The detailed molecular structure (atom positions, bond lengths, and angles) determined by both techniques also agreed closely.
Conclusion: The study confirmed that PXRD is an accurate and reliable method for figuring out the crystal structure of small organic molecules like griseofulvin. It serves as a strong alternative or complementary tool to SCXRD, particularly when it's difficult to grow large, high-quality single crystals.
 Figure 1. Comparison of griseofulvin crystal structure determined by (a) powder X-ray diffraction and (b) single-crystal X-ray diffraction. (Pan Q Q, et al., 2012)
Figure 1. Comparison of griseofulvin crystal structure determined by (a) powder X-ray diffraction and (b) single-crystal X-ray diffraction. (Pan Q Q, et al., 2012)
At Creative Biostructure, we offer comprehensive X-ray crystallography services and X-ray powder diffraction (XRPD) analysis tailored to your research and industrial needs. Whether you need high-resolution crystallographic data or a quick and reliable phase identification, our experts can assist you. Contact us to discuss your project and find the most suitable X-ray diffraction solution.
References
- Pan Q Q, Guo P, Duan J, et al. Comparative crystal structure determination of griseofulvin: powder X-ray diffraction versus single-crystal X-ray diffraction. Chinese Science Bulletin. 2012, 57: 3867-3871. https://doi.org/10.1007/s11434-012-5245-5
- Chauhan A, Chauhan P. Powder XRD technique and its applications in science and technology. J Anal Bioanal Tech, 2014, 5(5): 1-5. https://doi.org/10.4172/2155-9872.1000212
- Thakral N K, Zanon R L, Kelly R C, et al. Applications of powder X-ray diffraction in small molecule pharmaceuticals: achievements and aspirations. Journal of pharmaceutical sciences. 2018, 107(12): 2969-2982. https://doi.org/10.1016/j.xphs.2018.08.010
- Holder C F, Schaak R E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS nano. 2019, 13(7): 7359-7365. https://doi.org/10.1021/acsnano.9b05157