Membrane Protein Crystallization
Membrane proteins are proteins associated with the membranes. Membrane proteins have a variety of function, which are essential to biological process, playing important roles in enzymatic catalysis, signal transduction, communication of cell with their environment, transport and storage, control of metabolic process, and the control of growth and differentiation. Therefore, membrane proteins are interesting scientifically because of their key roles in controlling the processes of life. Each protein has a flexible conformation and has a specific binding and transformation of substrates. Now membrane proteins are the targets of most pharmaceuticals and approximately 60% of the current targets for drug discovery are membrane proteins. Even more critically, the dysfunction of membrane proteins has been involved with many diseases, such as diabetes, cirrhosis of the liver, cancer, Alzheimer's disease, hypertension, epilepsy, cataract, tubulopathy, leukodystrophy, Leigh syndrome, anemia, sensorineural deafness, and hypertrophic cardiomyopathy, though the structure of many of these proteins and the corresponding dysfunctions are not understood. Therefore, it is urgent to study the interactions between the structure and function of membrane protein for human disease therapy.
Crystallization is a technique that has long been used as a purification procedure because crystals naturally exclude contaminants. First a solution of the species of interest is made under conditions favoring dissolution. Crystallization is then achieved by altering the solution conditions so as to decrease the solubility of the desired product to the point where it will precipitate out of solution creating a solid crystalline form. This decrease in solubility is typically achieved by changes in temperature, concentration, or by the addition of a precipitating agent such as a salt, an osmotically active molecule such as polyethylene glycol, or an anti-solvent. The growth of high quality protein crystals for X-ray analysis is achieved in a similar fashion; first through the identification of crystal forming conditions, followed by optimization of crystallization conditions to produce the highest quality crystals. One of the main bottlenecks associated with protein crystallography is the identification of crystal forming conditions. However, no methods currently exist to predict crystallization conditions a priori. Now, people have developed a High-Throughput Crystallization Platform for rapid crystal forming conditions screening.
Expression, solubilization, and purification
Protein crystallization is one of the most important techniques to determine the structure of proteins, especially about interaction of the proteins with ligands and substrates. This technology follows the process of target protein expression, solubilization, and purification.
There are at least three factors that determine the crystallizability of membrane protein:
(1) The ability to extract and solubilize the protein from membrane environment;
(2) The stability of the mixed protein/detergent micelles under crystallization conditions;
(3) The purity and homogeneity of the targeted membrane protein sample.
These factors determine much of the extra biochemical experimentation for membrane protein crystallization.
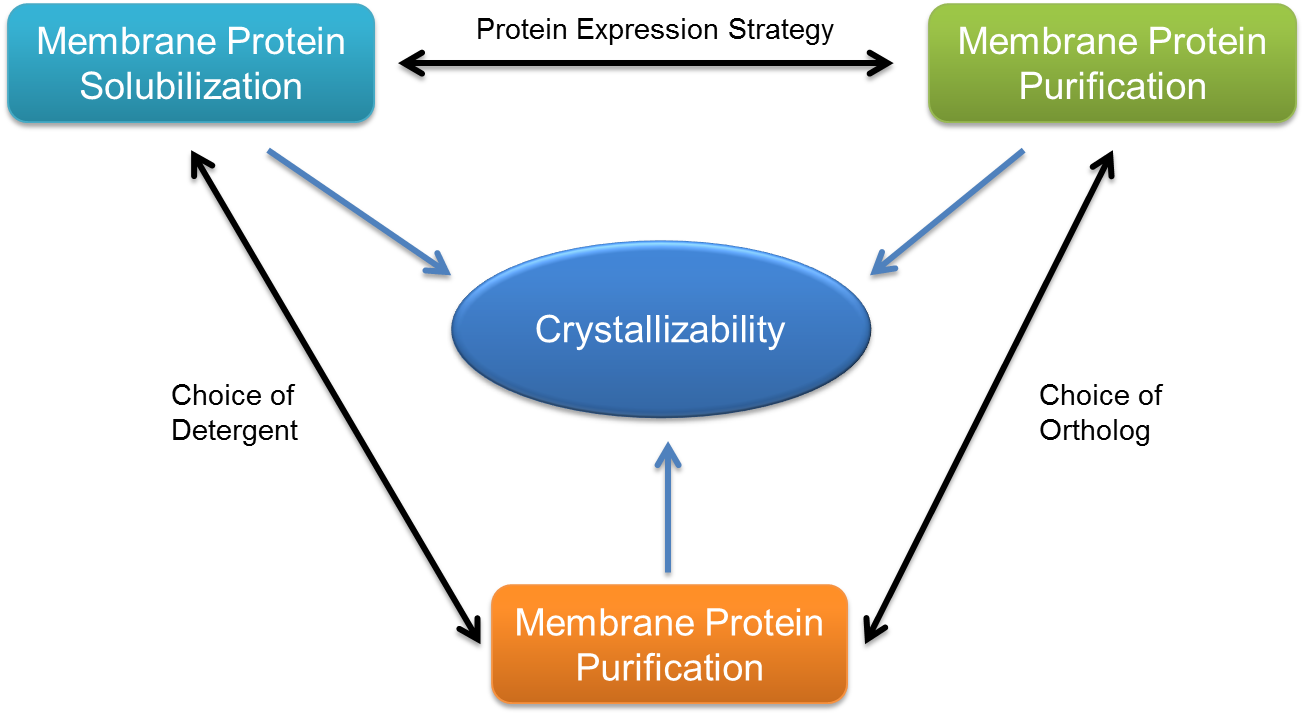 Figure 1. The crystallizability of every membrane protein.
Figure 1. The crystallizability of every membrane protein.
To get pure membrane protein, one suitable membrane protein expression system is needed. After identification of expression system, the next step is the solubilization of the target protein because membrane proteins are strongly membrane associated. In order to solubilize them, detergents such as SDS, Triton X-100 or alkylglycosides are used. The detergents which form micelles in water are small amphipathic molecules. If the membrane protein is mixed with detergents, the hydrophobic regions of the membrane proteins can be attached with the hydrophobic ends of detergents, which lead to displacement of the lipid molecules. This binding of the membrane proteins enable detergent-protein complexes to be soluble as small protein and lipid micellar clusters.
The challenge of membrane protein crystallizationThe amphiphilic nature of membrane proteins prevented the crystal formation. Amphiphilicity in particular is a challenge in crystallization, because protein crystal growth occurs from a solution of protein and dissolution occurs as an interaction of molecules with a like solvent (i.e. polar – polar or apolar – apolar). However, amphiphilic membrane proteins have both hydrophobic and hydrophilic portions they will not readily dissolve into either a polar or an apolar solvent on their own.
A variety of strategies have been used to solve the amphiphilicity of membrane protein, for example, the in surfo detergent solubilization method and the in meso method. The in surfo method encapsulates the hydrophobic portions of the membrane protein in a detergent micelle to allow for dissolution. But the in meso method maintains the membrane protein in a membrane-like environment. Despite these strategies, it is also very difficult to crystallize membrane proteins, Less than 1% structures deposited in the Protein Databank are for membrane proteins, with even fewer present at high resolution (< 2Å). Among these structures, the number of human membrane proteins is less than 20. Although the genomic analyses had indicating that membrane proteins should be similar in structure and function.
Crystallization
The next step crystallization of the membrane protein; this step involves detergent removal from micellar detergent solutions containing the purified protein and a suitable combination of lipids. The two dimensional method for crystallizing integral membrane proteins involves mixing a detergent solution of the purified protein with a detergent solution of the desired lipid. Excess detergent is then removed. As the detergent is removed, the lipid and membrane protein coalesce into membrane structures. Confinement of the membrane protein leads to increased encounter frequency and crystallization when conditions are favorable.
A large number of factors critically determine the production of crystals. In most cases, a relatively narrow range of protein concentrations, lipid : protein ratios, lipid types, detergent types, ionic strengths, pHs, and temperatures will affect 2D membrane protein crystallization. Also, detergent removal is a key step.
Reference
1. Copyright 2010 Sarah Louise Perry – Ideals
2. Nature Reviews Molecular Cell Biology 16, 69–81 (2015) doi:10.1038/nrm3933