Nuclear Skeleton Structure
The nuclear skeleton, also known as the nuclear matrix, is a dynamic and intricate network of proteins that provides structural support to the nucleus and plays a critical role in regulating nuclear architecture, gene expression, and chromatin organization. Despite its importance, the exact composition and functions of the nuclear skeleton remain the subject of ongoing research. The study of the nuclear skeleton is essential for understanding several cellular processes, including gene regulation, DNA replication, and cellular aging. The nuclear skeleton provides an elastic shell at the nuclear periphery and scaffolds for localized stiffness in the interior, both of which are thought to serve as platforms for various genomic functions. It is composed of lamins, nuclear actin, and a number of additional structural proteins (e.g., spectrins).
Nuclear Lamina
The nuclear lamina is a two-dimensional filamentous network at the periphery of the nucleus in higher eukaryotes, directly underlying the inner nuclear membrane. Nuclear lamina serves to maintain nuclear stability, organize chromatin and bind nuclear pore complexes (NPCs) and a steadily growing list of nuclear envelope proteins and transcription factors. The major components of nuclear lamina are lamins.
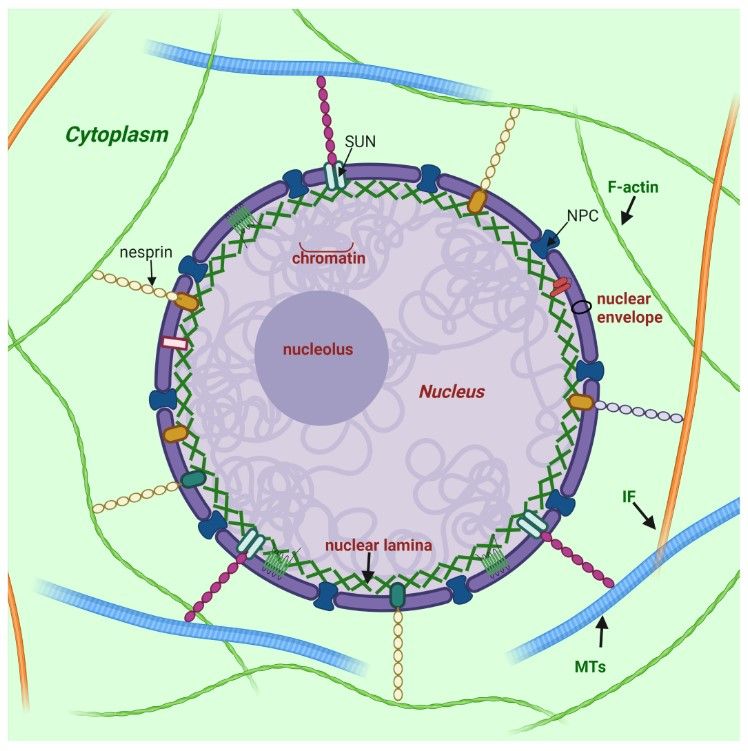 Figure 1. Nuclear lamina position and its interplay with other structures of cell (Malashicheva and Perepelina, 2021).
Figure 1. Nuclear lamina position and its interplay with other structures of cell (Malashicheva and Perepelina, 2021).
What Are Lamins and How Are They Structured?
Lamins are fibrous proteins in type V intermediate filaments that consist of a three-part domain structure: a head domain, a helical coiled-coil rod domain, and a tail domain. The α-helical rod domain consists of four segments, 1A, 1B, 2A, 2B, separated by linker segments. The coiled coils have a characteristic heptad pattern in which hydrophobic amino acid residues are located at the 1st and 4th positions of a seven amino acid segment. These hydrophobic amino acids form the coiled-coil structure. The C-terminal tail domain contains a nuclear localization signal (NLS), an immunoglobulin domain (Ig-like) and a conserved CAAX box that undergoes farnesylation. Notably, lamin C lacks codons for the final 82 amino acids, including the CaaX motif.
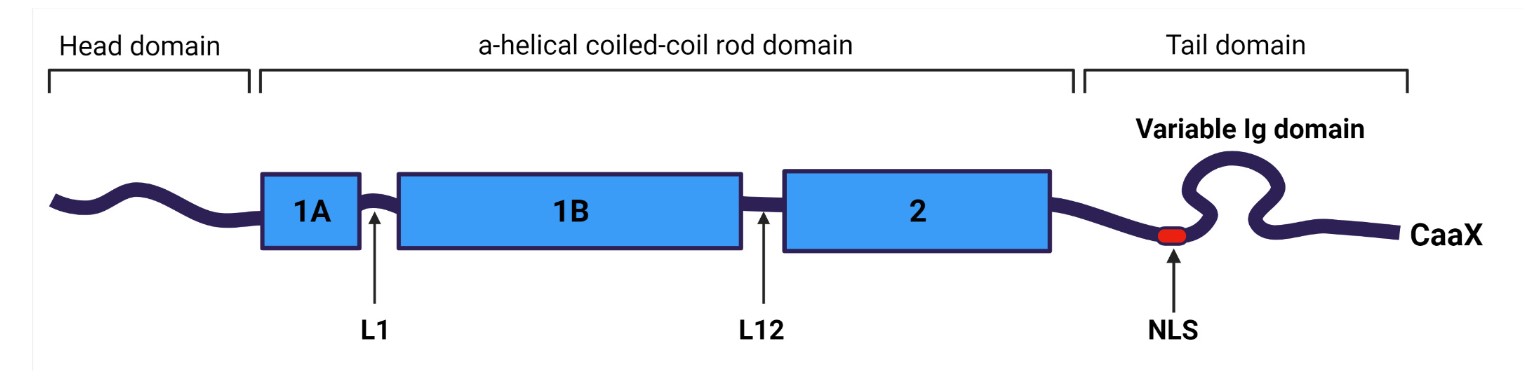 Figure 2. Common structure of lamins (Malashicheva and Perepelina, 2021).
Figure 2. Common structure of lamins (Malashicheva and Perepelina, 2021).
Lamins are divided into two main types: Type A and Type B lamins. A-type lamins (lamins A and C) are encoded by the LMNA gene and are expressed in differentiated cells. B-type lamins (lamins B1 and B2) are encoded by the LMNB1 and LMNB2 genes, respectively, and are essential for cell viability and are expressed in all cell types. In particular, the expression of A-type and B-type lamins is highly variable and depends on the cell type and differentiation stage. Structurally, A-type lamins share a common N-terminal domain and rod domain but have different C-terminal ends. Lamin C is shorter because it lacks the C-terminal CaaX sequence present in lamin A. B-type lamins share structural similarities with lamin A/C, such as the central rod domain, but have distinct N- and C-terminal sequences. The two lamin subtypes also undergo different post-translational modifications, with B-type lamins retaining the addition of a farnesyl group while A-type lamins do not.
Both A- and B-type lamins assemble into filaments of similar dimensions. Lamin dimers are formed from monomers that associate in parallel, forming a coiled coil through the central α-helical rod domain. Lamin dimers are then assembled head-to-tail to form protofilaments. Protofilaments form a polymer by antiparallel association of lamin polymers. Finally, multiple polymers cooperate to form a 10 nm long lamin filament.
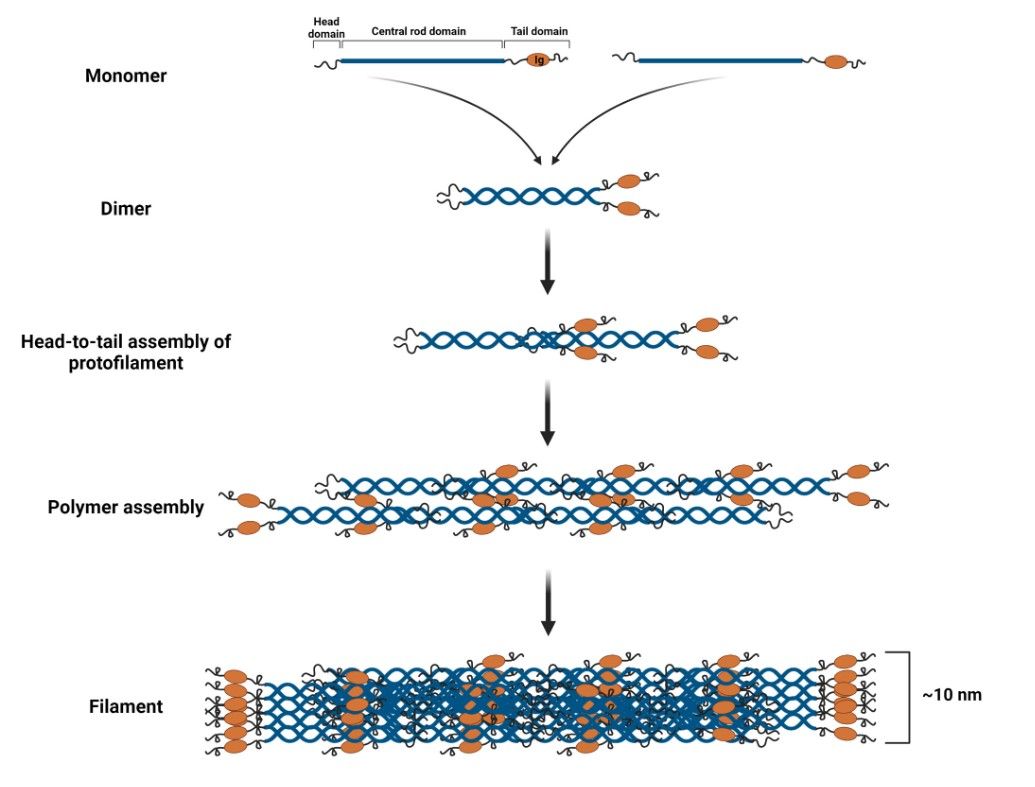 Figure 3. Lamin filament assembly (Malashicheva and Perepelina, 2021).
Figure 3. Lamin filament assembly (Malashicheva and Perepelina, 2021).
Other Nuclear Lamina Components
In addition to lamins, a variety of integral and peripheral membrane proteins contribute to nuclear lamina assembly. Important examples include lamina-associated polypeptides 1 and 2 (LAP1, LAP2), emerin, lamin B receptor (LBR), otefin, and MAN1. These proteins, located in or associated with the inner nuclear membrane, facilitate the attachment of the nuclear lamina to the nuclear envelope. In addition to nuclear membrane proteins, a number of transcription factors bind to the lamina, including retinoblastoma transcriptional regulator (RB), germ cell-less (GCL), sterol response element binding protein (SREBP1), FOS, and MOK2. Barrier to autointegration factor (BAF) is a chromatin-associated protein that also binds to the nuclear lamina and several of the nuclear envelope proteins mentioned above. Heterochromatin protein 1 (HP1) binds both chromatin and the LBR.
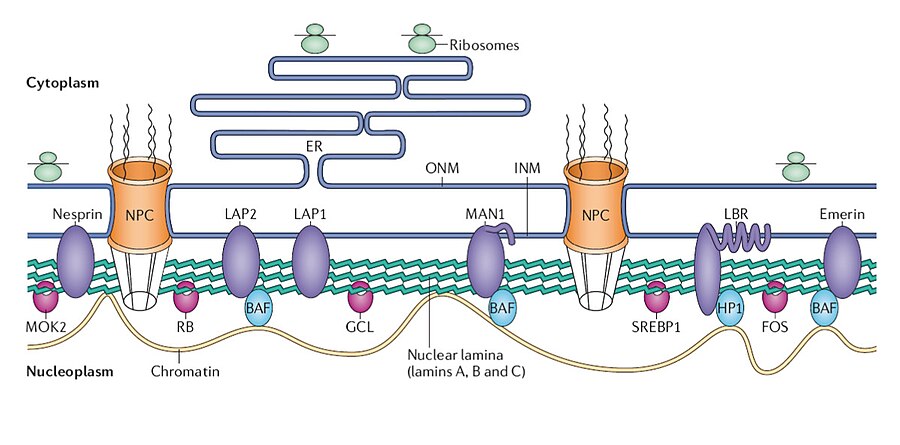 Figure 4. Structure of the nuclear lamina. Nuclear envelope proteins: purple; transcription factors: pink. ONM, outer nuclear membrane.
Figure 4. Structure of the nuclear lamina. Nuclear envelope proteins: purple; transcription factors: pink. ONM, outer nuclear membrane.
LINC Complex
Lamins associate with LINC (Linker of Nucleoskeleton and Cytoskeleton) complexes that span the nuclear envelope primarily through direct binding, with SUN (Sad1 and UNC-84) proteins in the inner nuclear membrane binding to the nuclear lamina and anchoring the LINC complex within the nuclear envelope. The KASH (Klarsicht, ANC-1, and Syne Homology) domain proteins in the outer nuclear membrane, by binding to cytoskeletal structures in the cytoplasm, provide an indirect link between the nuclear lamina and the cytoskeleton, facilitating the transmission of mechanical forces between the nucleus and the cytoplasm. This interaction is critical for mechanotransduction, whereby mechanical signals from the cytoskeleton are transmitted to the nucleus, influencing gene expression, nuclear positioning, and cellular responses to mechanical stress.
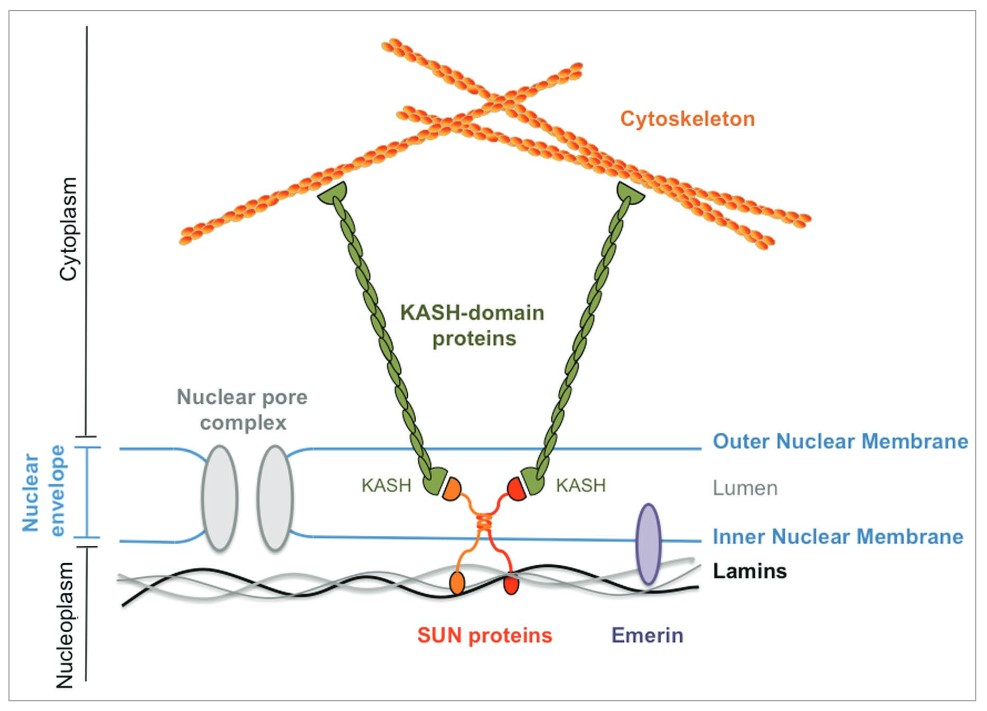 Figure 5. Prototypical LINC complex. SUN-domain proteins: red and orange; lamina: black and grey; emerin: purple; KASH-domain protein: green (Méjat and Misteli, 2010).
Figure 5. Prototypical LINC complex. SUN-domain proteins: red and orange; lamina: black and grey; emerin: purple; KASH-domain protein: green (Méjat and Misteli, 2010).
Nuclear Actin
Nuclear actin, a poorly understood component of the nuclear skeleton, has traditionally been associated with the cytoskeleton, but its presence and functions in the nucleus are of increasing interest. Unlike its cytoplasmic counterpart, nuclear actin exists in monomeric or oligomeric forms and is involved in several nuclear processes, including chromatin remodeling, transcriptional regulation, and maintenance of nuclear structure.
Spontaneous actin nucleation is characterized by a fast-growing (+) "barbed" end and a slow-growing (-) "pointed" end, with more efficient addition of G-actin to the (+) end. F-actin assembly and disassembly are regulated by actin remodelers, including nucleating, severing, capping, and cross-linking proteins. A number of proteins regulate actin dynamics.
The regulation of nuclear actin is facilitated by a range of proteins, including Arp2/3, which promotes nucleation; spire, which promotes elongation; formins, which promote polymerization; cofilin, which stimulates filament severing; and CapZ, which blocks filament elongation. The response of nuclear F-actin to various stimuli is distinct. In the context of DNA damage, formin- and Arp2/3-dependent filament formation is triggered for the purpose of repair. Conversely, in the presence of serum stimulation and cell spreading, MRTF-A is activated through formin-dependent filaments. Activation of the TCR and baculovirus infection both rely on Arp2/3-dependent filaments for cytokine expression and nuclear egress, respectively. Additionally, G1 phase cells depend on formin-induced actin polymerization for replication initiation and nuclear expansion.
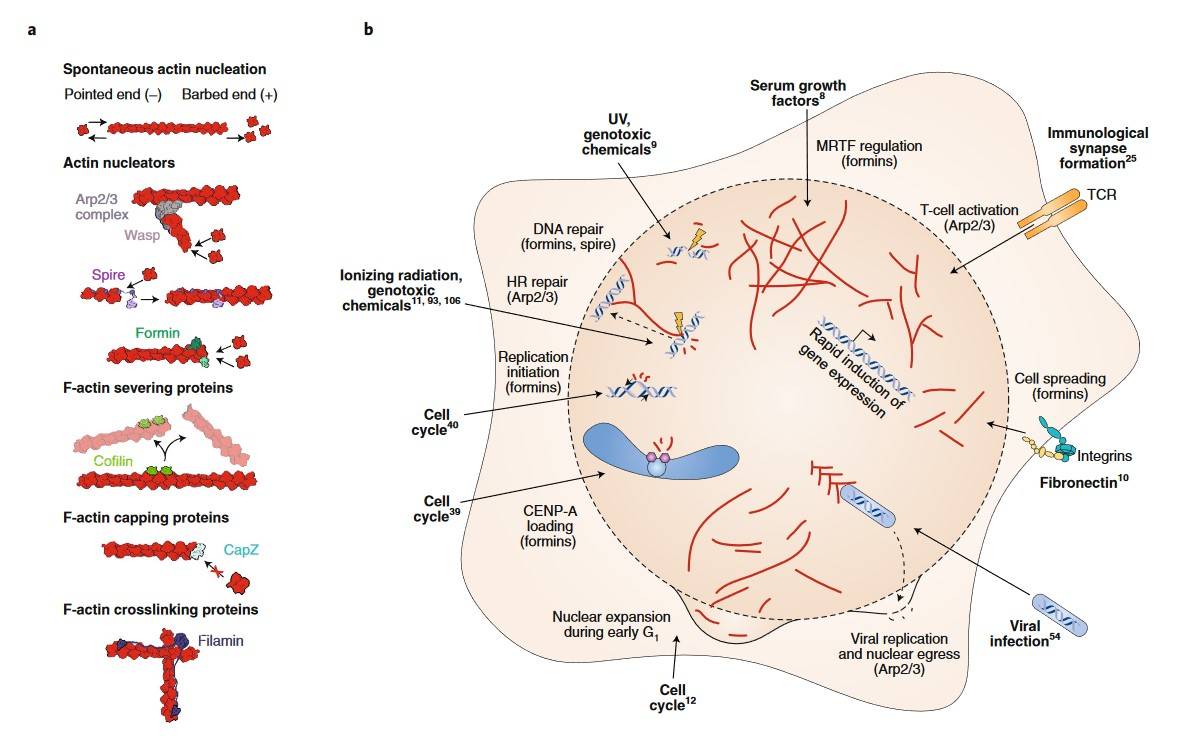 Figure 6. Different actin remodeling pathways are shown (Caridi et al., 2019).
Figure 6. Different actin remodeling pathways are shown (Caridi et al., 2019).
Other Nucleoskeletal Candidates
In addition to lamins and actin, several other proteins have been proposed as potential components of the nuclear skeleton. These include spectrins, which are known for their role in the cytoskeleton but have also been detected in the nucleus. Spectrins are thought to contribute to nuclear shape and stability and may be involved in the organization of chromatin.
Another candidate is titin, a giant protein best known for its role in muscle elasticity. Nuclear titin has been implicated in the mechanical stability of the nucleus and in the regulation of chromatin architecture. Other potential nucleoskeletal proteins include nesprins, which link the nuclear envelope to the cytoskeleton, and emerin, a nuclear envelope protein that interacts with lamins and is involved in nuclear architecture and gene expression regulation.
Methods for Analyzing Nuclear Skeleton Structure
A variety of methods are used to study the structure of the nuclear cytoskeleton, each providing different insights into its composition and function. One common method is immunofluorescence microscopy, which uses antibodies to detect specific nuclear proteins, allowing researchers to visualize their localization and organization within the nucleus. Another technique is electron microscopy, which provides high-resolution images of the nuclear architecture, revealing the intricate details of the nuclear lamina and other nucleoskeletal components. Biochemical fractionation, combined with mass spectrometry, is used to isolate and identify nuclear matrix proteins, providing insights into the protein composition of the nuclear skeleton. Advanced techniques such as super-resolution microscopy and cryo-electron tomography have also been employed to study the nuclear skeleton. These methods allow for the visualization of the nuclear architecture at unprecedented levels of detail, providing new insights into the spatial organization of nucleoskeletal components.
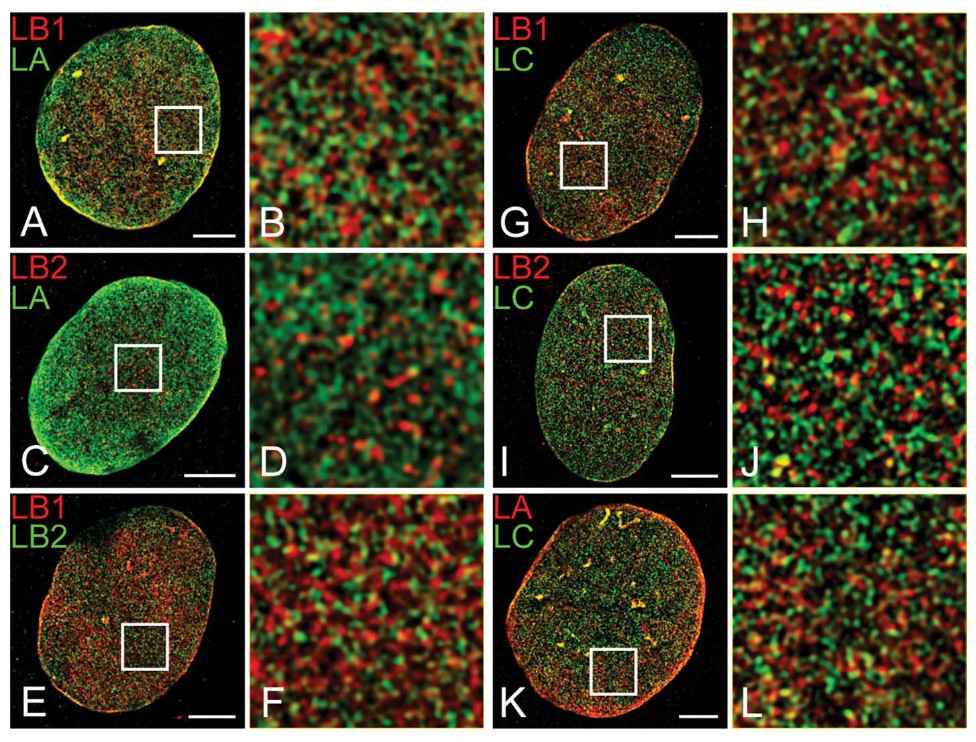 Figure 7. Colocalization of lamin isoforms in MEFs using indirect immunofluorescence and three-dimensional structured illumination microscopy (3D-SIM) (Shimi et al., 2015).
Figure 7. Colocalization of lamin isoforms in MEFs using indirect immunofluorescence and three-dimensional structured illumination microscopy (3D-SIM) (Shimi et al., 2015).
The nuclear skeleton is a complex and dynamic structure that is essential for maintaining nuclear integrity, regulating gene expression, and organizing chromatin. Components such as nuclear lamins, nuclear actin, and other nucleoskeletal candidates, along with nuclear motor proteins, work together to maintain the functional architecture of the nucleus.
Creative Biostructure offers advanced cryo-EM solutions for subcellular organelles to unravel the structure of the nuclear skeleton, providing a deeper understanding of its contributions to cellular function and disease. To discover how our services can enhance your nuclear skeleton structure research, contact us for a customized quote.
References
- Caridi, C. P., Plessner, M., Grosse, R., & Chiolo, I. (2019). Nuclear actin filaments in DNA repair dynamics. Nature Cell Biology, 21(9), 1068–1077.
- Evangelisti, C., Rusciano, I., Mongiorgi, S., Ramazzotti, G., Lattanzi, G., Manzoli, L., Cocco, L., & Ratti, S. (2022). The wide and growing range of lamin B-related diseases: From laminopathies to cancer. Cellular and Molecular Life Sciences, 79(2), 126.
- Malashicheva, A., & Perepelina, K. (2021). Diversity of nuclear lamin a/c action as a key to tissue-specific regulation of cellular identity in health and disease. Frontiers in Cell and Developmental Biology, 9, 761469.
- Méjat, A. (2010). LINC complexes in health and disease. Nucleus, 1(1), 40–52.
- Shimi, T., Kittisopikul, M., Tran, J., Goldman, A. E., Adam, S. A., Zheng, Y., Jaqaman, K., & Goldman, R. D. (2015). Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Molecular Biology of the Cell, 26(22), 4075–4086.
- Simon, D. N., & Wilson, K. L. (2011). The nucleoskeleton as a genome-associated dynamic "network of networks." Nature Reviews Molecular Cell Biology, 12(11), 695–708.
- Tenga, R., & Medalia, O. (2020). Structure and unique mechanical aspects of nuclear lamin filaments. Current Opinion in Structural Biology, 64, 152–159.