Structural Research of Diacylglyceryl Transferases
The biosynthetic pathway for bacterial lipoproteins, which is essential for bacterial survival, involves three reactions catalyzed by different enzymes. The first reaction is catalyzed by the membrane enzyme diacylglyceryl transferase (Lgt), which recognizes the lipobox motif in pre-prolipoproteins and transfers a diacylglyceryl group from phosphatidylglycerol to the conserved cysteine residue in the motif. The crystal structures of Escherichia coli Lgt with lipid ligands revealed a laterally opening central cavity with the active site and two binding sites for phosphatidylglycerol, the donor substrate. The structures also supported previous structure-function relationships of Lgt and identified critical residues, including Arg143 and Arg239, essential for diacylglyceryl transfer. Mutagenesis and in vitro studies identified critical residues essential for diacylglyceryl transfer and suggested a feasible mechanism of substrate recognition.
These studies of Lgt structure and the biosynthetic pathway for bacterial lipoproteins are crucial for understanding the fundamental biological functions of these molecules and developing new antibiotics to combat bacterial infections.
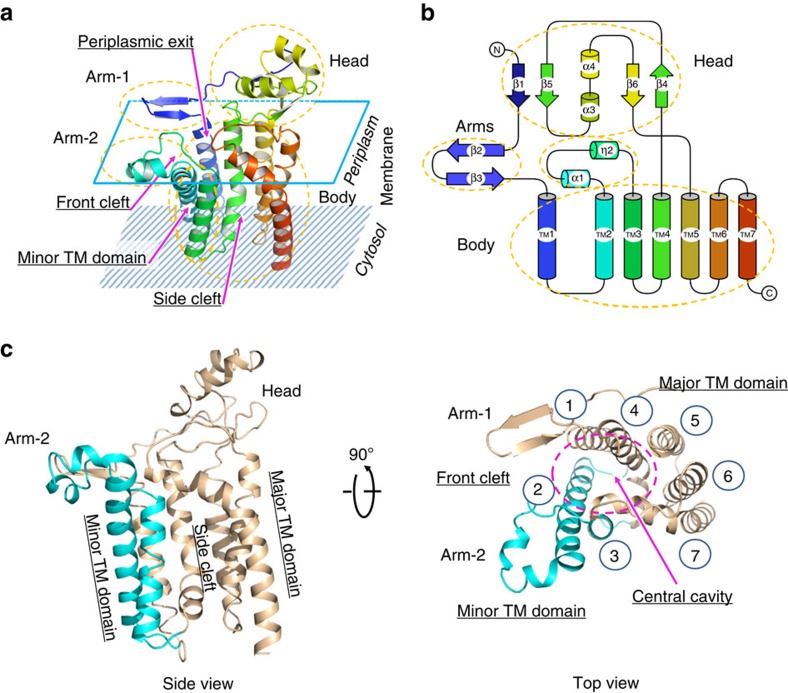 Figure 1. The overall structure of EcLgt. (Mao G, et al., 2016)
Figure 1. The overall structure of EcLgt. (Mao G, et al., 2016)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Phosphatidylglycerol: prolipoprotein diacylglyceryl transferase (Lgt) in complex with phosphatidylglycerol (expressed in E. coli) | Escherichia coli | X-ray diffraction | 1.90 Å | 5AZC |
Table 1. Structural Research of Diacylglyceryl Transferases.
As a pioneering company that leads structural analysis, we offer a comprehensive range of protein structural biology services. Our team of experienced structural biologists uses advanced techniques such as X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy to provide high-quality structural data that can be used to advance research in the field of lipid metabolism. We understand that each project is unique, which is why we offer customized solutions tailored to the specific needs of our clients. Our team works closely with you to understand your research objectives, ensuring that our services are aligned with your goals.
Our cutting-edge technology and expertise allow us to provide efficient and accurate analysis, providing you with a full analysis report of your enzyme's structure and function. Our high-quality structural data is critical for drug discovery and development, which is why we take every step to ensure that our clients receive the best service possible. Contact us today to learn more about our services, our team is here to answer any questions you may have and to help you get started on your project.
Reference
- Mao G, et al. Crystal structure of E. coli lipoprotein diacylglyceryl transferase. Nature Communications. 2016, 7(1): 10198.
