Reversed-Phase Chromatography (RPC)
Reversed-Phase Chromatography (RPC) is a widely used chromatographic technique in both analytical and preparative applications. It is best known for its ability to separate complex mixtures of biomolecules, including proteins, peptides, and small molecules. RPC is a subset of high-performance liquid chromatography (HPLC) and is characterized by unique separation mechanisms and applications.
What Is Reversed-Phase Chromatography?
Reversed-Phase Chromatography (RPC) is a liquid chromatography technique where the stationary phase is nonpolar, and the mobile phase is polar. Unlike normal phase chromatography, where the stationary phase is polar and the mobile phase is nonpolar, RPC operates in a reversed configuration. This technique is primarily used to separate compounds based on their hydrophobic interactions with the stationary phase.
Basic Principles and Workflow of RPC
In RPC, the stationary phase is composed of a nonpolar material, typically silica particles coated with hydrophobic chains, such as octyl (C8) or octadecyl (C18) groups. These hydrophobic chains provide the stationary phase with its nonpolar character. The mobile phase in RPC is a polar solvent or a mixture of polar solvents, such as water mixed with acetonitrile or methanol. The polarity of the mobile phase can be adjusted by changing the ratio of water to organic solvent, thereby affecting the interaction between the analytes and the stationary phase.
The separation mechanism in RPC is based on hydrophobic interactions between the analytes and the stationary phase. During the chromatographic process:
Sample Injection: The sample is introduced into the RPC column, where analytes interact with the hydrophobic stationary phase.
Retention: Molecules with higher hydrophobicity have stronger interactions with the nonpolar stationary phase and thus are retained longer.
Elution: The analytes are eluted by gradually increasing the polarity of the mobile phase, which decreases the hydrophobic interactions and allows the molecules to be washed off the column. Less hydrophobic molecules elute first, followed by more hydrophobic molecules.
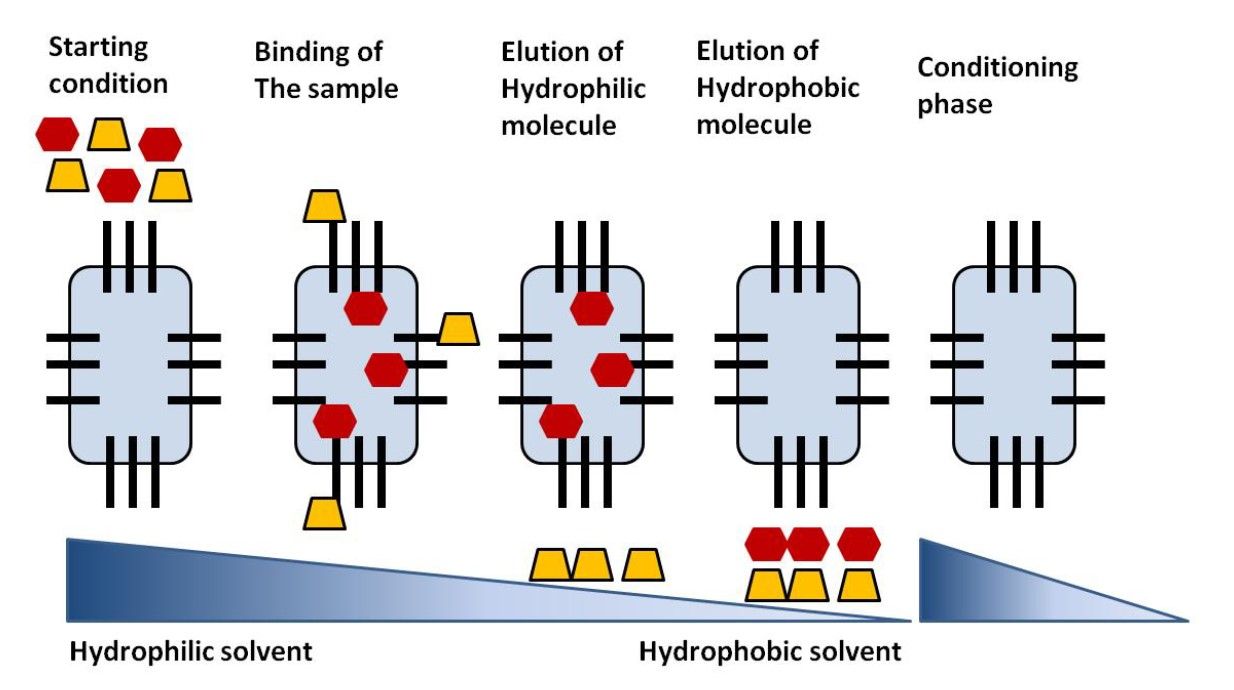 Figure 1. Steps of a of reversed-phase chromatography separation (Magdeldin and Moser, 2012).
Figure 1. Steps of a of reversed-phase chromatography separation (Magdeldin and Moser, 2012).
Why Is RPC More Common?
Reversed-Phase HPLC has become the most widely used mode of chromatography due to several factors:
Table 1. Advantages of RPC technique.
| Versatility | RPC is versatile and can handle a wide range of compounds, from small molecules to large biomolecules like proteins and peptides. Its ability to separate compounds based on hydrophobic interactions makes it applicable to many different types of samples. |
| High Resolution and Sensitivity | RPC provides high resolution and sensitivity, making it effective for separating complex mixtures. The use of nonpolar stationary phases and polar mobile phases allows for fine-tuning of separation conditions, resulting in high-resolution separations. |
| Wide Availability of Columns and Equipment | RPC columns and equipment are widely available and standardized, making it easy for researchers to adopt and implement the technique. The broad availability of different stationary phases and mobile phase compositions allows for customization based on specific analytical needs. |
| Reproducibility | RPC provides reproducible results, which is crucial for both analytical and preparative purposes. The consistency in separation and the ability to fine-tune conditions contribute to its reliability. |
| Ease of Use | The RPC technique is relatively straightforward and user-friendly. The method of increasing polarity in the mobile phase to elute analytes is well understood and easy to implement, making it accessible for a wide range of applications. |
Limitations of RPC
Despite these advantages, RPC has several limitations:
Table 2. Limitations of RPC technique.
| Limited to Hydrophobic Interactions | RPC is limited to separating compounds based on hydrophobic interactions. It may not be suitable for separating compounds that do not exhibit significant hydrophobic properties. |
| Sample Preparation | Samples may require appropriate solubilization in polar solvents before analysis. Some biomolecules may be challenging to solubilize, affecting the efficiency of RPC. |
| Column Lifetime | The nonpolar stationary phases used in RPC can be susceptible to degradation over time, especially with harsh solvents or high temperatures. Regular maintenance and proper handling are required to extend column lifetime. |
| Not Ideal for Highly Polar Compounds | RPC may not be effective for highly polar compounds that do not interact strongly with the hydrophobic stationary phase. Alternative chromatographic methods may be needed for such compounds. |
Applications of RPC
Protein Purification: RPC is extensively used for the purification of proteins. Proteins often have varying hydrophobic regions, which can be exploited to separate them from other proteins and contaminants. RPC helps in obtaining pure proteins for further structural analysis and functional studies.
Peptide Mapping: In structural biology, RPC is used for peptide mapping, which involves separating peptides derived from protein digestion. This technique is crucial for identifying peptide sequences and understanding protein structure and function.
Protein-Protein and Protein-Ligand Interactions: RPC can be employed to study protein-protein and protein-ligand interactions by analyzing changes in hydrophobic interactions. Understanding these interactions is essential for elucidating the mechanisms of biological processes and for drug discovery.
Characterization of Post-Translational Modifications: Post-translational modifications, such as phosphorylation or glycosylation, can alter the hydrophobic properties of proteins. RPC is used to analyze these modifications by separating modified and unmodified forms of proteins.
Analysis of Protein Folding and Stability: RPC is useful for studying protein folding and stability. Changes in hydrophobic interactions can provide insights into the folding process and the stability of different conformations, which is important for understanding protein function and dysfunction.
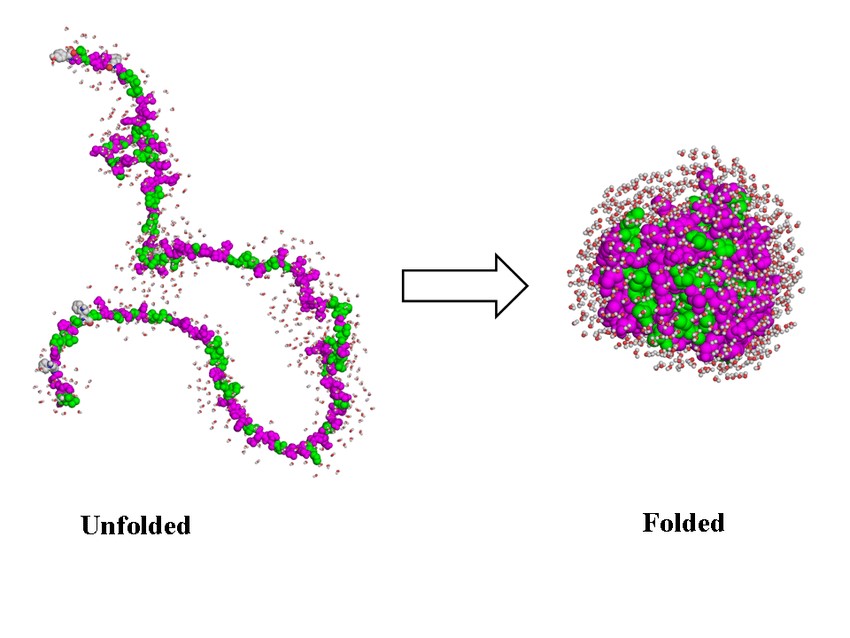 Figure 2. Protein folding: from primary to tertiary structure (Guidi et al., 2016).
Figure 2. Protein folding: from primary to tertiary structure (Guidi et al., 2016).
In conclusion, Reversed-Phase Chromatography (RPC) is a powerful and widely used technique in chromatography, offering high resolution, sensitivity, and versatility. By exploiting hydrophobic interactions between analytes and a nonpolar stationary phase, RPC efficiently separates a broad range of compounds. Compared to other chromatographic techniques, RPC's ease of use, reproducibility, and the availability of standardized columns contribute to its widespread adoption. In structural biology, RPC plays a crucial role in protein purification, peptide mapping, studying interactions, and characterizing post-translational modifications. Despite its limitations, RPC remains a fundamental tool in both research and industrial applications, providing valuable insights into complex biological systems.
At Creative Biostructure, we offer specialized Reversed-Phase Chromatography services, utilizing the latest technology and advanced equipment. Our experienced team is dedicated to delivering accurate and efficient separation of various macromolecules and biological structures. With our expertise in handling challenging samples, we provide customized solutions for protein, peptide, and other biomolecular separations. Whether you're conducting early-stage research or need precise analysis, our reliable services are designed to meet your specific needs. Contact us today to learn how our Reversed-Phase Chromatography expertise can elevate your scientific research.
References
- Lehninger Principles of Biochemistry, Fifth Edition, 2008. W.H. Freeman and Company.
- Colin, H., & Guiochon, G. (1977). Introduction to reversed-phase high-performance liquid chromatography. Journal of Chromatography A, 141(3), 289–312.
- Guidi, G., Di Tucci, L., & Santambrogio, M. D. (2016). ProFAX: A hardware acceleration of a protein folding algorithm. 2016 IEEE 2nd International Forum on Research and Technologies for Society and Industry Leveraging a Better Tomorrow (RTSI), 1–6.
- Josic, D., & Kovac, S. (2010). Reversed‐phase high performance liquid chromatography of proteins. Current Protocols in Protein Science, 61(1).
