Protein Homogeneity
In the domain of protein research and development, the homogeneity of a protein sample is paramount. Whether you are working on structural studies, therapeutic protein development, or quality control assays, the consistency and uniformity of your protein preparation can significantly impact the reliability of your results. At Creative Biostructure, we specialize in providing state-of-the-art protein structure analysis and quality control assay services designed to ensure the highest level of protein homogeneity.
What Is Protein Homogeneity?
Protein homogeneity refers to the consistent and uniform composition of a protein sample, wherein the molecular species present are identical or nearly identical in structure, size, and charge. In the context of biochemistry and molecular biology, ensuring protein homogeneity is of paramount importance for a wide array of applications, ranging from structural biology to drug development. A homogeneous protein sample is one that exhibits a uniform molecular weight, amino acid sequence, tertiary structure, and post-translational modifications. This level of uniformity is crucial for both qualitative and quantitative analyses, as well as for maintaining reproducibility in experimental results.
The Importance of Protein Homogeneity
Structural Biology
In structural biology, the need for homogeneous protein samples is equally compelling. High-resolution techniques, such as X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy (cryo-EM), require protein samples that are not only pure but also structurally homogeneous. For example, X-ray crystallography relies on the formation of well-ordered crystals, which are difficult to obtain if the protein sample is heterogeneous. Structural information derived from heterogeneous samples may be incomplete or misleading, impeding efforts to elucidate mechanisms of action or design inhibitors.
Disease Research
In addition, protein homogeneity is critical to understanding the molecular basis of disease. For example, in neurodegenerative diseases, aggregation of proteins such as amyloid-beta or tau is a hallmark of the pathology. In such cases, characterizing these aggregates at the molecular level requires an understanding of how protein homogeneity is disrupted. Dissecting the mechanisms behind protein misfolding, aggregation, and heterogeneity provides insight into disease progression and potential therapeutic interventions.
Biological Research
The importance of protein homogeneity extends far beyond basic research. In drug discovery and development, for example, obtaining a pure and homogeneous protein sample is essential to understanding its biological function and therapeutic potential. Therapeutic proteins, such as monoclonal antibodies or enzyme replacements, must be homogeneous to ensure consistent efficacy, safety and batch-to-batch reproducibility. Impurities or variants within these proteins can lead to undesirable effects, including altered pharmacokinetics, reduced potency, or immune responses. Therefore, protein homogeneity is a quality control measure in biopharmaceutical manufacturing that has a direct impact on product performance.

Methods for Assessing Protein Homogeneity
A variety of analytical techniques are used to evaluate protein homogeneity. These methods fall into two broad categories: techniques for assessing the purity of the protein sample and techniques for assessing the uniformity of the structural characteristics of the protein. The following are some of the most commonly used methods for evaluating protein homogeneity.
Gel Electrophoresis
Gel electrophoresis, specifically sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), is a standard method for assessing protein purity and homogeneity. In SDS-PAGE, proteins are denatured and loaded onto a polyacrylamide gel. Under an electric field, proteins migrate according to their molecular weight, allowing researchers to determine the presence of contaminants or degradation products. A single, sharp band on the gel indicates a homogeneous protein sample, while multiple bands indicate heterogeneity. However, this technique provides only size information and does not directly assess the conformation or structural integrity of the protein.
Size-Exclusion Chromatography (SEC)
Size-Exclusion Chromatography (SEC), also known as gel filtration chromatography, separates proteins based on their size. Homogeneous proteins will elute as a single peak, whereas heterogeneous samples will produce multiple peaks corresponding to different molecular species. SEC can also provide insights into the oligomerization state of the protein, as well as its overall size distribution. This is particularly useful for assessing the homogeneity of proteins that tend to form aggregates or multimers, such as membrane proteins or large enzyme complexes.
Dynamic Light Scattering (DLS)
Dynamic light scattering (DLS) is a technique that measures the size distribution of particles in solution by analyzing the fluctuations in scattered light caused by Brownian motion. DLS is particularly sensitive to the presence of aggregates or oligomers in protein samples (Protein Aggregation Analysis). Homogeneous proteins will have a narrow particle size distribution, whereas heterogeneous samples will have a broader distribution, indicating protein aggregation or conformational heterogeneity. DLS can be used to monitor the stability of proteins over time and under various experimental conditions.
Ultracentrifugation
Analytical ultracentrifugation (AUC) is another powerful technique for assessing protein homogeneity. In AUC, proteins are subjected to high-speed centrifugal forces, which cause them to sediment according to their size, shape, and density. The resulting sedimentation profiles provide detailed information about the molecular weight and oligomeric state of the protein. Homogeneous proteins will produce a single, well-defined peak, whereas heterogeneous samples will produce multiple peaks or shoulders. AUC is also useful for detecting conformational changes, such as folding intermediates or denatured states, that may not be evident by other methods.
Mass Spectrometry (MS)
Mass spectrometry (MS) is an invaluable tool for assessing the molecular composition and purity of protein samples. MS allows researchers to precisely determine the mass of individual protein molecules, providing detailed information about post-translational modifications, sequence variants, and heterogeneity at the molecular level. Techniques such as liquid chromatography-mass spectrometry (LC-MS) and electrospray ionization (ESI-MS) are particularly useful for identifying and quantifying contaminants, truncations, or modifications that may affect the protein's homogeneity.
Cryo-Electron Microscopy (Cryo-EM)
In structural biology, cryo-Electron Microscopy (cryo-EM) has emerged as a powerful tool for visualizing protein structures at near-atomic resolution. The success of cryo-EM depends on the homogeneity of the protein sample, as the technique relies on the collection of thousands of individual particle images to generate a 3D reconstruction. Heterogeneity can manifest itself as blurred or poorly resolved regions in the final map, limiting the accuracy of the structural model. To address this, cryo-EM data acquisition protocols often include a thorough assessment of particle homogeneity prior to data acquisition.
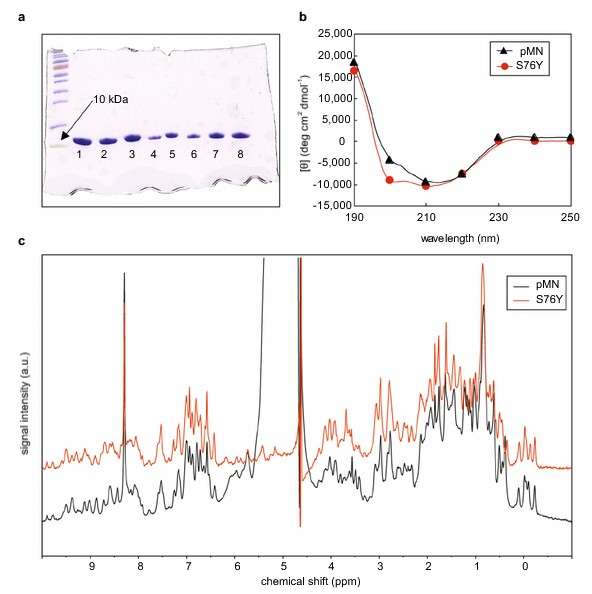 Figure 1. An example of protein homogeneity and structural analysis of single chain parent monellin and mutants. (a) SDS-PAGE. (b) Far UV spectra. (c) Far UV spectra. (Weiffert and Linse, 2018)
Figure 1. An example of protein homogeneity and structural analysis of single chain parent monellin and mutants. (a) SDS-PAGE. (b) Far UV spectra. (c) Far UV spectra. (Weiffert and Linse, 2018)
Protein homogeneity is a fundamental aspect of protein research, therapeutic development, and quality control. At Creative Biostructure, we understand the importance of achieving consistent, high-quality protein preparations and offer a range of advanced services to ensure that your protein samples meet the highest standards of homogeneity. Contact us today to learn more about how our protein structure analysis and quality control assays can enhance the success of your next project.
References
- Raynal B, Brûlé S, Uebel S, Knauer SH. Assessing and improving protein sample quality. In: Daviter T, Johnson CM, McLaughlin SH, Williams MA, eds. Protein-Ligand Interactions. Vol 2263. Springer US; 2021:3-46.
- Weiffert T, Linse S. Protein stabilization with retained function of monellin using a split GFP system. Sci Rep. 2018;8(1):12763.
