Structural Research of Cellulose Synthases
Cellulose synthesis is carried out by complex multi-subunit enzymes called cellulose synthases, which are found in a wide range of organisms, including bacteria, algae, and plants. Understanding the structure and function of cellulose synthases is essential for improving our ability to manipulate cellulose synthesis for various applications. In recent years, there have been significant advances in the structural research of cellulose synthases, which have provided new insights into the mechanisms of cellulose synthesis.
A recent study used cryo-electron microscopy (cryo-EM) to determine a poplar cellulose synthase CesA homotrimer at near-atomic resolution and combined it with biochemical and biophysical assays to characterize its catalytic activity. The study showed that the plant cellulose synthase complex is composed of three identical subunits that form a homotrimeric structure, and identified the specific amino acid residues that are critical for cellulose synthesis. This study provides valuable insights into the architecture and function of the plant cellulose synthase complex, which could have implications for our understanding of cellulose synthesis in other organisms and for the development of plant-based biomaterials.
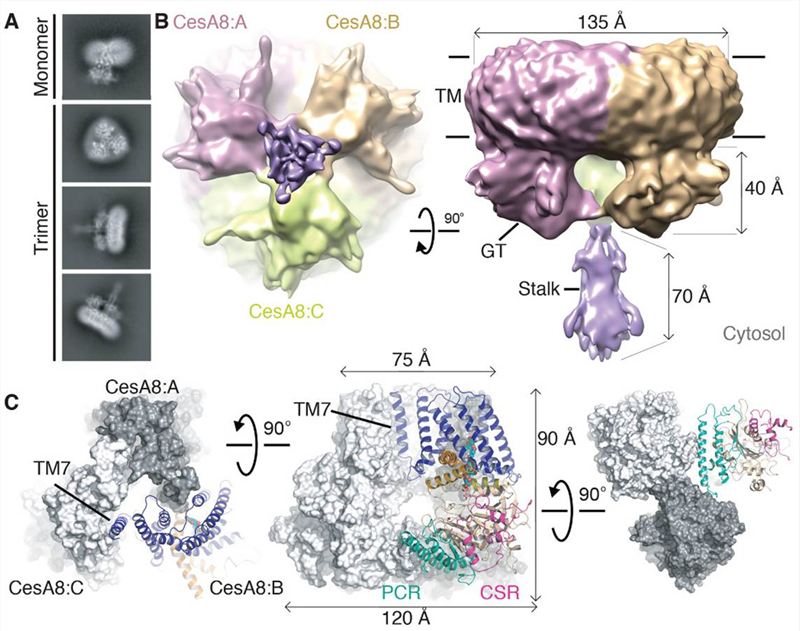 Figure 1. Cryo-EM structure of PttCesA8. (Purushotham P, et al., 2020)
Figure 1. Cryo-EM structure of PttCesA8. (Purushotham P, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| BcsA-BcsB cellulose synthase/cellulose translocation intermediate (expressed in E. coli) | Cereibacter sphaeroides | X-ray diffraction | 3.25 Å | 4HG6 |
| BcsB cellulose synthase hexamer (expressed in E. coli) | Escherichia coli | Cryo-EM single particle analysis | 3.40 Å | 7L2Z |
| CesA homotrimer involved in cellulose microfibril formation (expressed in Spodoptera frugiperda) | Populus tremula | Cryo-EM single particle analysis | 3.50 Å | 6WLB |
| CesA7 cotton cellulose synthase isoform 7 (expressed in HEK293 cells) | Gossypium hirsutum | Cryo-EM single particle analysis | 3.50 Å | 7D5K |
| Hyaluronan synthase (expressed in E. coli) | Paramecium bursaria Chlorella virus CZ-2 | Cryo-EM single particle analysis | 3.10 Å | 7SP6 |
Table 1. Structural Research of Cellulose Synthases.
Creative Biostructure stands at the forefront of pioneering companies that lead the industry in the provision of cutting-edge structural analysis services. With a team of exceptional experts who specialize in the utilization of advanced techniques such as cryo-EM, X-ray crystallography, and NMR spectroscopy, we are well-equipped to gain a profound understanding of the complex and intricate structure and function of cellulose synthases.
Our clients can enjoy the benefits of our comprehensive structural analysis services, which start from the initial stages of protein expression and purification and continue through to the final stages of structure determination. With an unwavering commitment to delivering high-quality, accurate, and prompt structural analysis results, Creative Biostructure remains unparalleled in the industry, consistently earning us a sterling reputation among our clients.
Whether you seek to learn more about our structural analysis services or wish to collaborate with us on a research project, we encourage you to contact us today. Our unwavering commitment to quality and excellence, combined with our expertise and experience, make us the premier choice for all your structural analysis needs.
References
- Acheson J F, et al. Molecular organization of the E. coli cellulose synthase macrocomplex. Nature Structural & Molecular Biology. 2021, 28(3): 310-318.
- Maloney F P, et al. Structure, substrate recognition and initiation of hyaluronan synthase. Nature. 2022, 604(7904): 195-201.
- Purushotham P, Ho R, Zimmer J. Architecture of a catalytically active homotrimeric plant cellulose synthase complex. Science. 2020, 369(6507): 1089-1094.
