Structural Research of GPI-Anchored Cell-Wall Proteins (GPI-CWPs)
Glycosylphosphatidylinositol (GPI) anchor is a post-translational modification used in eukaryotes to attach proteins to membranes. In fungi, most GPI ankyrin (GPI-AP) transfer from the plasma membrane (PM) to the cell wall, so they are called GPI anchored cell wall proteins (GPI-CWP). In ascomycete fungi, GPI-CWP has multiple functions, such as serving as an adhesin, enzyme, or cell wall integrity sensor. Dfg5 is a protein from the GH76 family in budding yeast and Candida albicans. A key step in cell wall biogenesis is the transfer of GPI ankyrin from the plasma membrane to the glycan network under the catalysis of Dfg5 enzyme.
Researchers reported the crystal structure of a fungal GH76 protein from the Dfg5 subfamily. They analyzed the structure of Dfg5 from filamentous fungi and used it for screening crystalline glycan fragments to reassemble GPI core glycans in a U-shaped conformation within their binding pockets.
The crystal structure of Dfg5 from C. thermophilum has been determined with a resolution of 1.05 Å. The 12 α-helices between helices 5/6 and 7/8 and two antiparallel β-strands form the (α/α)6 helical barrel fold of CtDfg5, which is very similar to the bacterial GH76 direct homologue with rmsd values of 3.0 and 3.1 Å for the 327 and 345 equivalent Cα positions, respectively. In contrast to the bacterial GH76 homologue, the C terminus of CtDfg5 does not terminate at α 12, but rather spans the rear of the structural domain before an additional helical segment begins to form on the other side. This additional helix is very close to a segment of the α3-α4 loop (P124 to S130), which shows increased flexibility depending on its B-factor and electron density. This extended segment is adjacent to the DD motif (D134/D135), which is a highly conserved catalytic marker for all known GH76 homologs.
The substrate binding state of Ct Dfg5 has also been characterized, providing theoretical support for understanding the mechanism of Dfg5-type transglycosidases. With the exception of inositol, all other hexoses of the core glycan can be unambiguously identified by the electron density in the CtDfg5-binding pocket. Mannose binds only to the -2 subsite of CtDfg5. In CtDfg5-α-1,2-mannose, the position of the central -2 mannose is further extended to subsite -3 by a second α-1,2-linked mannose. ctDfg5-α-1,6- mannobiose occupies subsites -2 and -1, the positions next to the active site. Finally, the structure of CtDfg5-glucosamine reveals the position of the final GPI core glycan component at subsite +1.
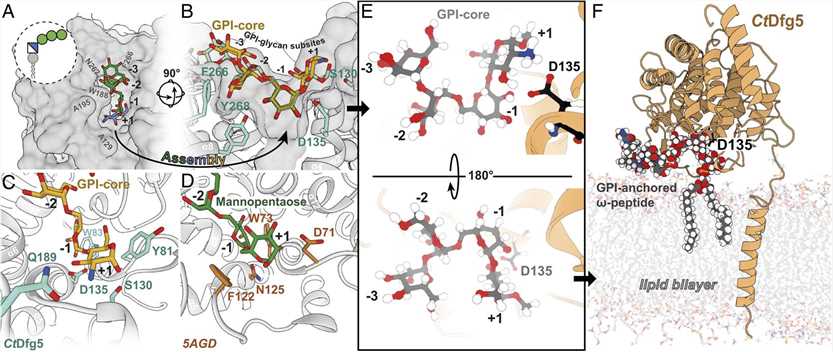 Figure 1. Reassembled GPI-core glycan in the binding pocket of Dfg5. (VOGT M S, et al., 2020)
Figure 1. Reassembled GPI-core glycan in the binding pocket of Dfg5. (VOGT M S, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Dfg5 GH76 protein | Thermochaetoides thermophila DSM 1495 | X-ray diffraction | 1.05 Å | 6RY0 |
| Dfg5 GH76 protein in complex with mannose | Thermochaetoides thermophila DSM 1495 | X-ray diffraction | 1.30 Å | 6RY1 |
| Dfg5 GH76 protein in complex with alpha-1,2-mannobiose | Thermochaetoides thermophila DSM 1495 | X-ray diffraction | 1.30 Å | 6RY2 |
| Dfg5 GH76 protein in complex with alpha-1,6-mannobiose | Thermochaetoides thermophila DSM 1495 | X-ray diffraction | 1.30 Å | 6RY5 |
| Dfg5 GH76 protein in complex with glucosamine | Thermochaetoides thermophila DSM 1495 | X-ray diffraction | 1.30 Å | 6RY6 |
| Dfg5 GH76 protein in complex with laminaribiose | Thermochaetoides thermophila DSM 1495 | X-ray diffraction | 1.30 Å | 6RY7 |
Table 1. Structural Research of GPI-CWPs.
Creative Biostructure provides customers with membrane protein analysis services based on X-ray crystallography technology. X-ray crystallography is a method to study the structure of materials by using X-ray diffraction technology. It can be used to determine the structure of macromolecules such as molecules, proteins, DNA, and RNA, and analyze their properties and functions, such as drug design and biomaterial research. X-ray crystallography is characterized by high resolution, high throughput, non-destructive, wide application range and low cost of consumables. If you are interested in learning more about our protein structural analysis services, please contact us for more information.
Reference
- VOGT M S, et al. Structural base for the transfer of GPI-anchored glycoproteins into fungal cell walls. Proceedings of the National Academy of Sciences, 2020, 117(36): 22061–22067.
