Structural Research of Energy-Coupling Factor Transporters
Energy-coupling factor (ECF) transporters are a group of small, asymmetric membrane protein complexes that play a vital role in the import of micronutrients in prokaryotes. These complexes consist of a substrate-binding protein (S component) embedded in the membrane and a tripartite ATP-hydrolyzing module (ECF module). ECF transporters have a highly unusual transport mechanism, which involves the toppling motion of the S component to drag the substrate across the membrane. In recent years, there has been significant progress in the structural research of ECF transporters. Researchers have used various techniques, such as cryogenic electron microscopy (cryo-EM), and X-ray crystallography, to determine the structures of ECF transporters and provide insights into their mechanism of action.
X-ray crystal structures have suggested that the S component of the complex must topple within the membrane to bring the substrate from the outside to the inside of the cell. Cryo-EM has shown to be a suitable method to study the structure of ECF transporters embedded in a more native environment, providing experimental evidence for a bilayer-mediated toppling mechanism. One recent study used cryo-EM to determine the structures of a folate-specific ECF transporter in lipid nanodiscs and detergent micelles at 2.7- and 3.4-Å resolution, respectively. The structures revealed an irregularly shaped bilayer environment around the membrane-embedded complex, suggesting that toppling of the S component is facilitated by protein-induced membrane deformations. These recent studies have provided valuable insights into the structure and mechanism of ECF transporters, which could lead to the development of new strategies to target these transporters for the treatment of bacterial infections.
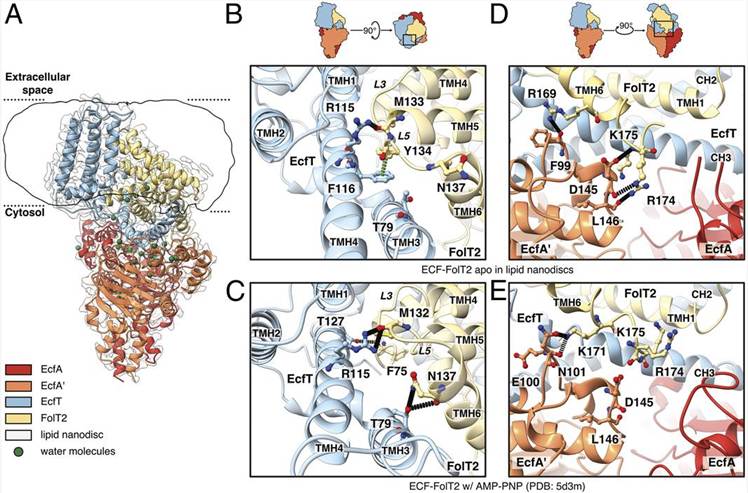 Figure 1. Cryo-EM structure of ECF-FolT2 in lipid nanodiscs. (Thangaratnarajah C, et al., 2021)
Figure 1. Cryo-EM structure of ECF-FolT2 in lipid nanodiscs. (Thangaratnarajah C, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| RibU, S Component of the Riboflavin Transporter (expressed in E. coli) | Staphylococcus aureus | X-ray diffraction | 3.60 Å | 3P5N |
| ThiT, S component of the Thiamin Transporter (expressed in Lactococcus cremoris) | Lactococcus cremoris | X-ray diffraction | 2.00 Å | 3RLB |
| BioY, S component of the Biotin Transporter (expressed in Lactococcus lactis) | Lactococcus cremoris | X-ray diffraction | 2.09 Å | 4DVE |
| Folate ECF transporter complex (expressed in E. coli) | Levilactobacillus brevis | X-ray diffraction | 3.00 Å | 4HUQ |
| ECF transporter complex (expressed in E. coli) | Levilactobacillus brevis | X-ray diffraction | 3.53 Å | 4HZU |
| Folate ECF transporter, apo form (expressed in E. coli) | Lactobacillus delbrueckii | X-ray diffraction | 3.00 Å | 5JSZ |
| Folate ECF transporter complex in MSP2N2 lipid nanodiscs (expressed in E. coli) | Lactobacillus delbrueckii | Cryo-EM single particle analysis | 2.70 Å | 7NNU |
| ECF-PanT energy-coupling factor pantothenate transporter (expressed in E. coli) | Levilactobacillus brevis | X-ray diffraction | 3.23 Å | 4RFS |
| ECF-PanT energy-coupling factor pantothenate transporter in complex with a nanobody (expressed in E. coli) | Lactobacillus delbrueckii | X-ray diffraction | 2.80 Å | 6ZG3 |
| BtuM cobalamin transporter (expressed in E. coli) | Thiobacillus denitrificans | X-ray diffraction | 2.01 Å | 6FFV |
Table 1. Structural Research of Energy-Coupling Factor Transporters.
Creative Biostructure is a leading provider of top-quality structural analysis services such as X-ray crystallography, NMR spectroscopy, and cryo-EM. Our cryo-EM technique is particularly effective for studying the structures of macromolecular complexes, especially membrane proteins like ECF transporters, thanks to its ability to preserve their native structure under cryogenic conditions. Our team of highly experienced scientists uses cutting-edge equipment and techniques to generate high-quality cryo-EM data and provide accurate structural analysis. Apart from cryo-EM, we also offer nanodiscs construction services that can be utilized to study the interactions of ECF transporter complexes with lipid bilayers in a native environment. Nanodiscs are self-assembling phospholipid bilayers that form disc-shaped structures. With our nanodiscs construction services, we provide a versatile tool for studying the structure and function of ECF transporter complexes in a more native lipid environment.
At Creative Biostructure, we value the satisfaction of our clients and always strive to provide the highest quality structural analysis services that meet and exceed their research needs. Our team works closely with clients to ensure that their expectations are met in terms of quality, accuracy, and turnaround time. If you are interested in exploring the structural research of ECF transporters and would like to learn more about our services, please feel free to contact us.
References
- Setyawati I, et al. In vitro reconstitution of dynamically interacting integral membrane subunits of energy-coupling factor transporters. Elife. 2020, 9: e64389.
- Thangaratnarajah C, et al. Insights into the bilayer-mediated toppling mechanism of a folate-specific ECF transporter by cryo-EM. Proceedings of the National Academy of Sciences. 2021, 118(34): e2105014118.