Structural Research of Immune Receptors
The immune system plays a pivotal role in defending the body against foreign invaders and maintaining overall health. Key components of the immune system are immune receptors, such as the αβ T cell receptor (TCR), which are essential for T cell development, activation, and immune responses to antigens. The intricate structure of immune receptors has been a subject of intense research, and recent breakthroughs in structural biology have provided unprecedented insights into their assembly and function.
Unveiling the Complexity: Cryo-EM Reveals the TCR-CD3 Complex
The TCR-CD3 complex is a critical mediator of T cell activation and immune responses. However, until recently, the mechanism of assembly of this complex has remained elusive. Through the innovative use of cryo-electron microscopy (cryo-EM), researchers have made groundbreaking strides in this area, and present a high-resolution cryo-EM structure of the human TCRαβ in complex with the CD3 hexamer. The detailed structure includes the complete extracellular domains and transmembrane helices of the TCR-CD3 complex, providing a comprehensive view of its organization at the molecular level.
Assembly Mechanism Unraveled: Key Findings
The octameric TCR-CD3 complex assembles with a precise 1:1:1:1 stoichiometry of TCRαβ:CD3γε:CD3δε:CD3ζζ. The extracellular domains of TCR-CD3 are mediated by the constant domains and connecting peptides of TCRαβ, forming a trimer-like structure in close proximity to the plasma membrane. These domains interact with CD3γε-CD3δε, stabilizing the overall complex.
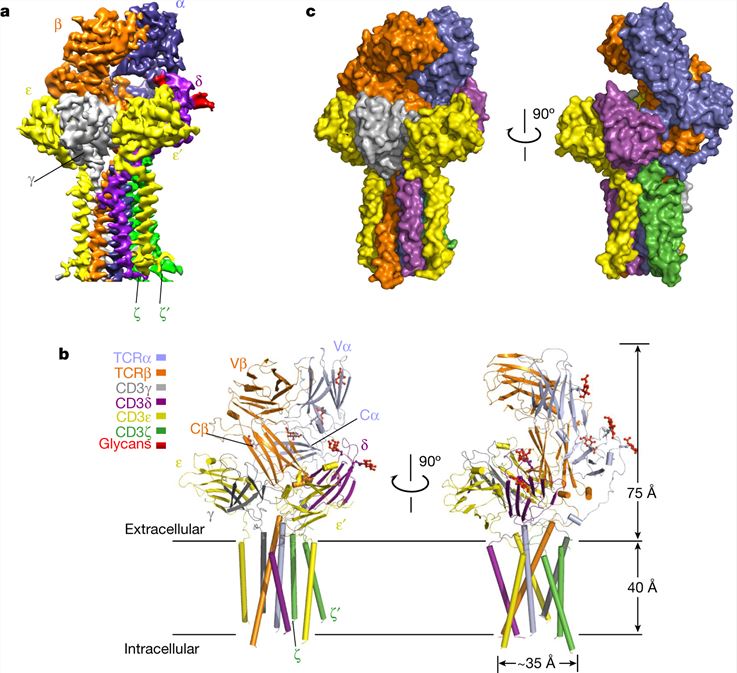 Figure 1. 3D reconstruction and atomic model of the human TCR-CD3 complex. (Dong D, et al., 2019)
Figure 1. 3D reconstruction and atomic model of the human TCR-CD3 complex. (Dong D, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| human T cell receptor-CD3 complex (expressed in Homo sapiens) | Homo sapiens | Cryo-EM single particle analysis | 3.70 Å | 6JXR |
| Zeta-Zeta Transmembrane Dimer (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 2HAC |
| membrane protein (WT) (expressed in Homo sapiens) | Homo sapiens | Cryo-EM single particle analysis | 3.20 Å | 7FJD |
| DAP12-NKG2C transmembrane heterotrimer (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 2L35 |
| DAP12 transmembrane domain in lipidic cubic phase (expressed in Escherichia coli) | Homo sapiens | X-ray diffraction | 1.77 Å | 4WOL |
| transmembrane domain of the CD28 dimer (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 7VU5 |
Table 1. Structural research of immune receptors.
As a pioneer in structural biology research, Creative Biostructure offers cutting-edge services that cater to the needs of researchers and pharmaceutical companies worldwide. Our structural analysis services provide detailed insights into the molecular architecture of various biological molecules, including immune receptors.
Using state-of-the-art techniques such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy (cryo-EM), our team of experts can elucidate the three-dimensional structures of immune receptors, helping to unravel their functional mechanisms and interactions. Contact us to discover the potential of our capabilities, which can enhance your research endeavors and move you closer to achieving your scientific goals.
References
- Dong D, et al. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. 2019, 573(7775): 546-552.
- Chen Y, et al. Cholesterol inhibits TCR signaling by directly restricting TCR-CD3 core tunnel motility. Molecular Cell. 2022, 82(7): 1278-1287. e5.
- Wu H, et al. Structural characterization of a dimerization interface in the CD28 transmembrane domain. Structure. 2022, 30(6): 803-812. e5.