Mempro™ Liposome Preparation by Reverse Phase Evaporation
Creative Biostructure provides a full range of products and services based on our unparalleled Mempro™ Liposome platform. Our featured Mempro™ Liposome Preparation services involve a variety of mechanical and non-mechanical methods, among which reverse phase evaporation technique is a generally employed one.
The reverse phase evaporation technique is composed of inverted micelles or water-in-oil emulsions in which the water phase contains the interested drugs and the organic phase consists of the lipids to form liposomal bilayer. More...
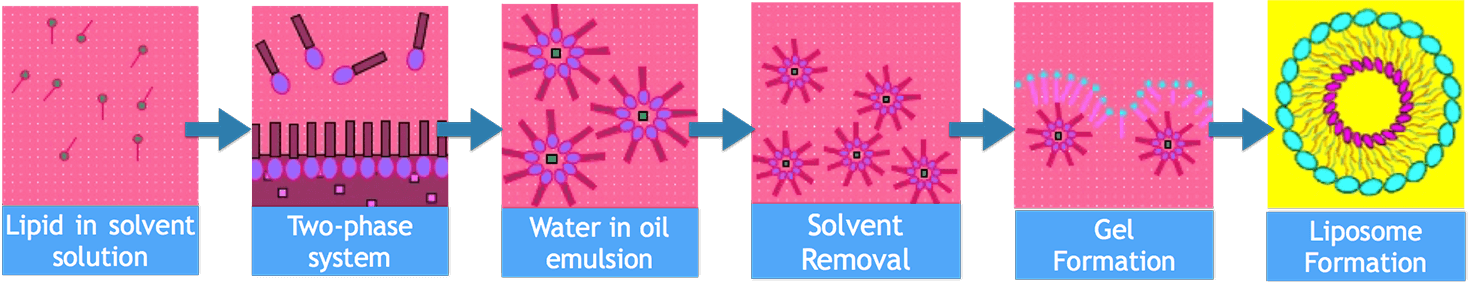 Figure 1. Flow Chart of Reverse Phase Evaporation
Figure 1. Flow Chart of Reverse Phase Evaporation
The CO2 can be acted as a convenient solvent at supercritical conditions since it is dense and non-condensable. Therefore, supercritical CO2 (scCO2) was introduced into the novel supercritical reverse phase evaporation method to dissolve the lipids. More...
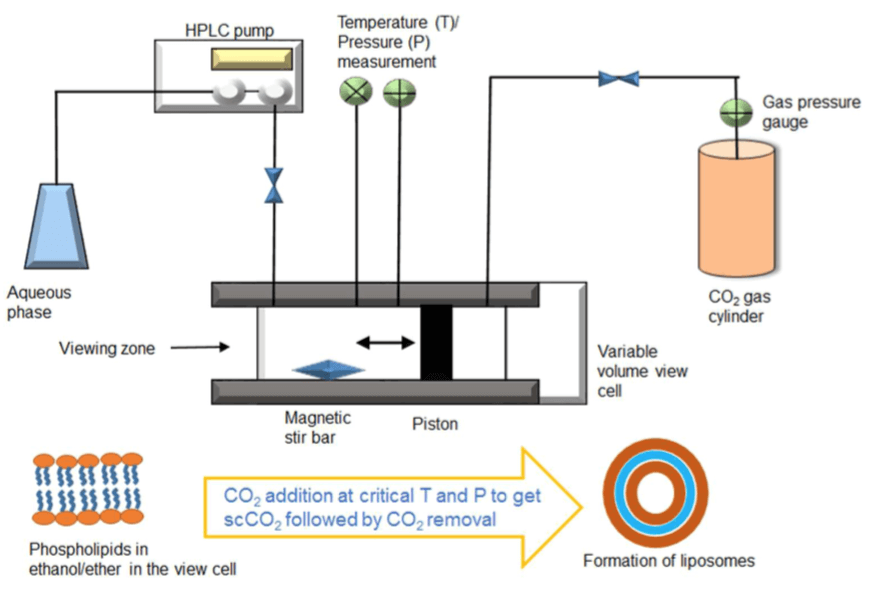 Figure 2. Schemed diagram of novel supercritical reverse phase evaporation (SRPE). Supercritical CO2 (scCO2) was introduced into the SRPE method to dissolve the phospholipids. (B. S. Pattni, et al., 2015)
Figure 2. Schemed diagram of novel supercritical reverse phase evaporation (SRPE). Supercritical CO2 (scCO2) was introduced into the SRPE method to dissolve the phospholipids. (B. S. Pattni, et al., 2015)
Advantages of SPRE:
- economic;
- environment-friendly;
- no contacts between the drugs and organic solvent
Besides reverse phase evaporation method, Creative Biostructure provides a full package of liposome preparation services based on our cutting-edge Mempro™ Liposome Technology. We have a group of rich-experienced scientific elites to ensure the best products and services for your custom demands. Creative Biostructure aims to be your reliable research partner. Please feel free to contact us for a detailed quote.
References:
G. Corace, et al. (2014). Multifunctional liposomes for nasal delivery of the anti-Alzheimer drug tacrine hydrochloride. Journal of Liposome Research, 24(4): 323-335.
B. S. Pattni, et al. (2015). New Developments in Liposomal Drug Delivery. Chemical Reviews, 115 (19): pp 10938–10966.
R. S. Kalhapure, et al. (2015). Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. Journal of Pharmaceutical Sciences, 104(3): 872-905.
