Structural Research of G Protein-coupled Receptors (GPCRs) Class B1
G Protein-coupled Receptors (GPCRs) are a superfamily of membrane proteins that play crucial roles in cell signaling and are essential targets for pharmaceutical research and drug development. Among the different classes of GPCRs, class B1 GPCRs have gained significant attention due to their involvement in mediating various physiological processes.
The GLP-1R (Glucagon-like peptide-1 receptor) is a prominent example of a class B1 GPCR, known for its role in glucose metabolism and regulation of insulin secretion. Deciphering the structure of class B1 GPCRs is crucial to understand their mechanisms of activation and signaling specificity. Recent advancements in cryo-electron microscopy (cryo-EM) have revolutionized the structural analysis of GPCRs, allowing researchers to visualize the receptor-ligand interactions at unprecedented resolutions.
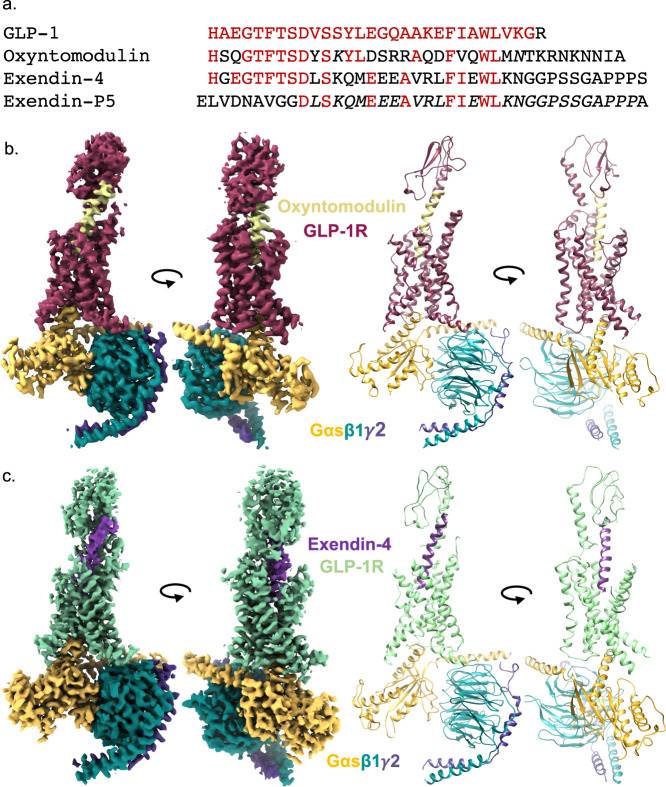 Figure 1. Cryo-EM structures of GLP-1R:Gs complexes with different agonists. (Deganutti G, et al., 2022)
Figure 1. Cryo-EM structures of GLP-1R:Gs complexes with different agonists. (Deganutti G, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Corticotropin-releasing factor receptor 1 (CRF1R) in complex with CP-376395 small-molecule antagonist (expressed in Trichoplusia ni) | Homo sapiens | X-ray diffraction | 2.98 Å | 4K5Y |
| Urocortin 1-bound Corticotropin-releasing factor 1 receptor in complex with Gs protein and Nb35 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.00 Å | 6PB0 |
| CRF1 Receptor Gs GPCR protein complex with CRF1 peptide (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.91 Å | 6P9X |
| Urocortin 1-bound Corticotropin-releasing factor 2 receptor in complex with Gs protein and Nb35 (expressed in Spodoptera frugiperda, Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 2.80 Å | 6PB1 |
| Corticotropin releasing factor receptor 2 bound to Urocortin 1 and coupled with heterotrimeric G11 protein (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.70 Å | 7TRY |
| PAC1 GPCR Receptor complex (expressed in Trichoplusia ni, Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 3.01 Å | 6P9Y |
| PAC1 receptor coupled to an engineered heterotrimeric G protein (expressed in Homo sapiens, Spodoptera frugiperda, Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 3.90 Å | 6LPB |
| PAC1 receptor in complex with PACAP38 (expressed in Insect BA phytoplasma) | Homo sapiens | Cryo-EM single particle analysis | 3.50 Å | 6M1I |
| Glucagon G protein coupled receptor (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 3.30 Å | 4L6R |
| Full-length glucagon receptor (GCGR) in complex with antagonist MK-0893 (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.50 Å | 5EE7 |
| Full-length glucagon receptor (GCGR) in complex with a truncated peptide (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 3.70 Å | 5NX2 |
| Full-length glucagon class B G protein-coupled receptor (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 3.00 Å | 5XEZ |
| Full-length glucagon receptor (GCGR) in complex with heterotrimeric GS protein (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 6B3J |
| Glucagon receptor (GCGR), full length in complex with glucagon analog NNC1702 (expressed in Sf9 cells) | Homo sapiens | X-ray diffraction | 3.00 Å | 5YQZ |
| Glucagon receptor in complex with Gs (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.70 Å | 6LMK |
| Full-length glucagon receptor (GCGR) in complex with P15-GCGR-Gs (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.40 Å | 6WHC |
| GCGR-Gs signaling complex bound to a designed glucagon derivative (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.10 Å | 6WPW |
| GIPR/GLP-1R/GCGR triagonist peptide 20-bound human GCGR-Gs complex (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.50 Å | 7V35 |
| Activated Glucagon-like peptide-1 receptor in complex with G protein (expressed in Spodoptera frugiperda) | Oryctolagus cuniculus | Cryo-EM single particle analysis | 4.10 Å | 5VAI |
| GLP-1 receptor complex with PF-06372222 (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.70 Å | 5VEW |
| GLP-1 receptor membrane domain, thermostabilized (M10) (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.80 Å | 6KJV |
| GLP-1 receptor in complex with TT-OAD2 non-peptide agonist (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.00 Å | 6ORV |
| Full length human GLP1 receptor in complex with Fab fragment (Fab7F38) (expressed in CHO-S cells) | Homo sapiens | X-ray diffraction | 3.20 Å | 6LN2 |
| GLP-1R-Gs complex structure with a small molecule full agonist (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 4.20 Å | 7C2E |
| GLP-1R-Gs complex with GLP-1 peptide and a positive allosteric modulator (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 6VCB |
| GLP-1 peptide hormone bound to Glucagon-Like peptide-1 (GLP-1) Receptor (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.10 Å | 6X18 |
| GLP-1R bound to non-peptide agonist LY3502970 (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.10 Å | 6XOX |
| Semaglutide-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in Complex with Gs protein (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.50 Å | 7KI0 |
| PF 06882961 bound to the glucagon-like peptide-1 receptor (GLP-1R) (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.24 Å | 7LCK |
| Compound 2-bound human GLP-1 receptor-Gs complex (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 7DUR |
| Peptide-19 bound to the Glucagon-Like Peptide-1 Receptor (GLP-1R) (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.14 Å | 7RTB |
| Oxyntomodulin-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in complex with Gs protein (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 7LLY |
| Ex4-D-Ala bound to the glucagon-like peptide-1 receptor/g protein complex (conformer 1) (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.41 Å | 7S1M |
| Glucagon-like peptide-1 receptor (GLP-1R)-Gs complex with bound tirzepatide (LY3298176) (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.40 Å | 7FIM |
| Boc5-bound hGLP-1R-Gs complex (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.61 Å | 7X8R |
| Glucagon-like peptide-1 receptor (GLP-1R) in complex with PF-06882961 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.80 Å | 7S15 |
| Glucagon-like peptide-2 receptor-Gs protein complex (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.00 Å | 7D68 |
| GIP insulinotropic polypeptide receptor (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.98 Å | 7DTY |
| GIP insulinotropic polypeptide receptor-Gs complex with bound tirzepatide (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.40 Å | 7FIY |
| Calcitonin receptor-heterotrimeric Gs protein complex (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 4.10 Å | 5UZ7 |
| Calcitonin receptor-heterotrimeric Gs bound to endogenous peptide and canonical transducer (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 6E3Y |
| Calcitonin receptor-heterotrimeric Gs protein complex (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.34 Å | 6NIY |
| Calcitonin gene-related peptide receptor (GPCR), apo form (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.15 Å | 7KNT |
| Amylin1 Receptor in complex with Gs and rat amylin peptide (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.20 Å | 7TYF |
| Parathyroid hormone receptor-1 (PTH1R) in complex with a parathyroid hormone analog and G protein, state 1 (expressed in Spodoptera aff. frugiperda 2 RZ-2014, Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 3.00 Å | 6NBF |
| Parathyroid hormone receptor-1 (PTH1R) in complex with a peptide agonist (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.50 Å | 6FJ3 |
| Parathyroid hormone receptor-2 (PTH2R) in complex with a tuberoinfundibular peptide of 39 residues and G protein (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.80 Å | 7F16 |
| Adrenomedullin 1 (AM1) receptor G protein complex with adrenomedullin peptid (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.00 Å | 6UUN |
| Adrenomedullin 2 (AM2) receptor G protein complex with adrenomedullin peptide (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.40 Å | 6UUS |
| Human secretin receptor Gs complex (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.30 Å | 6WZG |
| Human SECR in complex with an engineered Gs heterotrimer (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.90 Å | 7D3S |
| Vasoactive intestinal polypeptide 1 (VIP1) receptor-G protein complex (activated) (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.20 Å | 6VN7 |
| Vasoactive intestinal polypeptide 1 (VIP1) receptor-Gs complex with bound PACAP27 (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 2.30 Å | 8E3Y |
| Vasoactive intestinal polypeptide 2 (VIP2) receptor-Gs complex with bound PACAP27 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.74 Å | 7VQX |
| Growth hormone-releasing hormone receptor-Gs protein complex (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.60 Å | 7CZ5 |
Table 1. Structural research of class B1 GPCRs.
Creative Biostructure stands out as a leader in structural biology and GPCR research, boasting years of experience in the industry. Our commitment to excellence and cutting-edge technologies have enabled us to deliver high-quality structural information to clients worldwide. We have successfully determined the structures of several class B1 GPCRs, including the GLP-1R, bound to their endogenous agonists and coupled to G proteins. These structures have provided valuable insights into the molecular basis of receptor activation and biased agonism observed in GLP-1R agonists.
Our team of skilled scientists employs advanced techniques, such as cryo-electron microscopy (cryo-EM) and X-ray crystallography to acquire high-resolution structural data, offering invaluable revelations into the architecture and functionality of class B1 GPCRs. Contact us to explore how our advanced capabilities can enhance your research and bring you closer to accomplishing your scientific objectives.
References
- Deganutti G, et al. Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation. Nature Communications. 2022, 13(1): 92.
- Zhou F, et al. Structural basis for activation of the growth hormone-releasing hormone receptor. Nature Communications. 2020, 11(1): 5205.