Structural Research of Hydroxylases
Hydroxylase is a type of enzyme widely present in organisms, which can catalyze the addition or oxidation of hydroxyl groups in chemical reactions. Hydroxylase is an enzyme that catalyzes the addition of hydroxyl groups to substrates during oxidation reactions. In addition, hydroxylase can alter its chemical properties by adding a hydroxyl group to a toxic substance, thereby reducing its toxic effects on organisms. Thirdly, catalyze the synthesis of many metabolites in the organism (such as hormones, vitamin D, etc.). Finally, catalyze the oxidation reaction of certain chemical substances, thereby generating other useful molecules.
X-ray crystallography has been successfully applied to the structural study of phenylalanine hydroxylase, Prolyl 4-hydroxylases, and some deoxysaccharide hydroxylase and their complexes. With the help of X-ray crystallography, scientists were able to clarify the catalytic role of hydroxylase in biosynthesis and the substrate recognition mechanism.
For example, 4-proline hydroxylase (P4Hs) is widely present in eukaryotes and some prokaryotes, and P4Hs may be involved in the growth and development of Phytophthora and its interaction with host plants. In 2018, researchers obtained the protein crystal of pepper in Phytophthora capsici P4H1 (PcP4H1) through a prokaryotic expression system and successfully performed the structural analysis at a resolution of 2.35 Å. The three-dimensional structure shows that PcP4H1 contains the classic double-spanned β-helix (DSBH) core domain. Ferrous ions and cofactor are located in a larger active pocket. Loose β2-β3 loops, C-terminal α9-α10 loops and βII-βIII thumb loops may participate in the formation of substrate binding channels and determine the specificity of substrate binding.
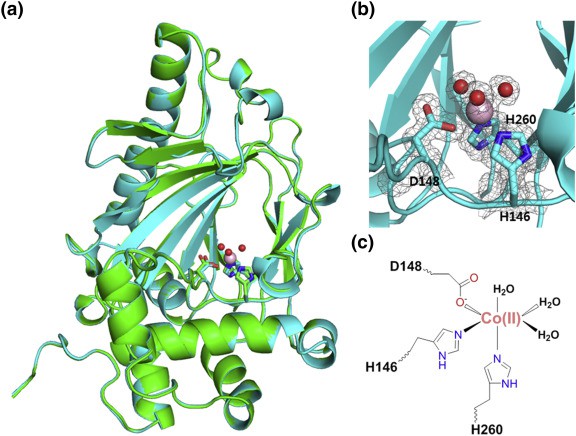 Figure
1. KdoOMI/Co(II) complex structure. (JOO, et al., 2018)
Figure
1. KdoOMI/Co(II) complex structure. (JOO, et al., 2018)
Fe(II)/α-Ketoglutarate/O2-Dependent Dioxygenase, Kdo 3-Hydroxylase (KdoO) is a membrane-related deoxyglucose hydroxylase that has no significant sequence identity with any known enzyme, and its structural information has not been reported before 2018. In addition, the researchers also used X-ray crystallography to characterize the structure of KdoO and its complex for the first time with a resolution of 1.45-1.94 Å. By characterizing the complex structures of KdoO, KdoO MI/α-KG/Fe (III), and KdoO MI/succinate/Fe (III) that bind to apolipoprotein and Co (II), researchers have identified the transformation of KdoO into Ko Kdo containing lipid A during the biosynthesis of lipopolysaccharides (LPS).
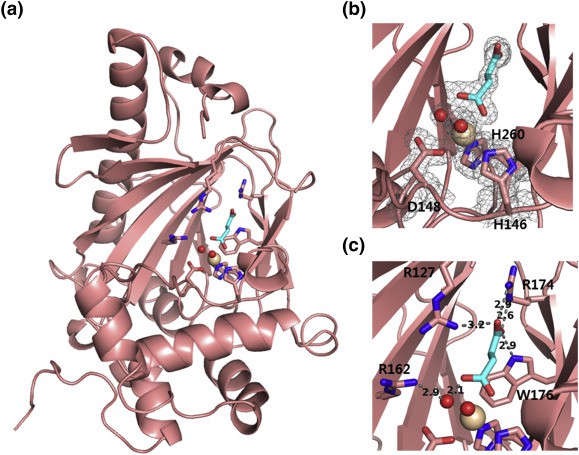 Figure 2. KdoOMI/succinate/Fe(III) complex (salmon) (JOO, et al., 2018)
Figure 2. KdoOMI/succinate/Fe(III) complex (salmon) (JOO, et al., 2018)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Kdo hydroxylase KdoO | Methylacidiphilum infernorum V4 | X-ray diffraction | 1.94 Å | 6A2E |
| Kdo hydroxylase KdoO in complex with cobalt (II) | Methylacidiphilum infernorum V4 | X-ray diffraction | 1.45 Å | 5YKA |
| Kdo hydroxylase KdoO in complex with alphaketoglutarate and Fe (III) | Methylacidiphilum infernorum V4 | X-ray diffraction | 1.6 Å | 5YVZ |
| Kdo hydroxylase KdoO in complex with succinate and Fe (III) | Methylacidiphilum infernorum V4 | X-ray diffraction | 1.49 Å | 5YW0 |
Table 1. Structural research of hydroxylases.
For scientists studying protein structure, X-ray crystallography is a very effective tool. It analyzes the molecular structure through diffraction patterns, providing a strong technical support for the study of various biomolecules. When analyzing the three-dimensional structure and function of membrane proteins and other biological macromolecules, X-ray crystallography technology shows its unique advantages, which also makes this technology make an important contribution to the field of life science. Creative Biostructure is a company specializing in the field of protein structural analysis. We can use high-intensity X-ray and detectors to provide excellent resolution and help scientists obtain more accurate and detailed structural data.
Reference
- JOO S H, et al. Biochemical and structural insights into an Fe (II)/α-ketoglutarate/O2-dependent dioxygenase, kdo 3-hydroxylase (KdoO). Journal of Molecular Biology, 2018, 430(21): 4036–4048.