Exosomes Loading Drugs by Extrusion
Exosomes are biologically active vesicles released by living cells into the extracellular microenvironment. Its diameter size is 30~150nm, the exosome membrane consists of the phospholipid bilayer, the surface carries membrane molecules from cell-specific, and the interior is rich in lipids, proteins nucleic acids, etc. The main function of exosomes is to transmit inter-cellular molecular information through their contents, and thus they have been widely researched in the field of drug delivery and continue to be highly popular. As drug carriers, exosomes have higher stability and biocompatibility than existing delivery systems for targeted therapies, and how to choose drug delivery strategies is an essential part of the clinical application. In recent years, the development of extrusion technology has led to a greater breakthrough in the efficiency of exosome drug loading.
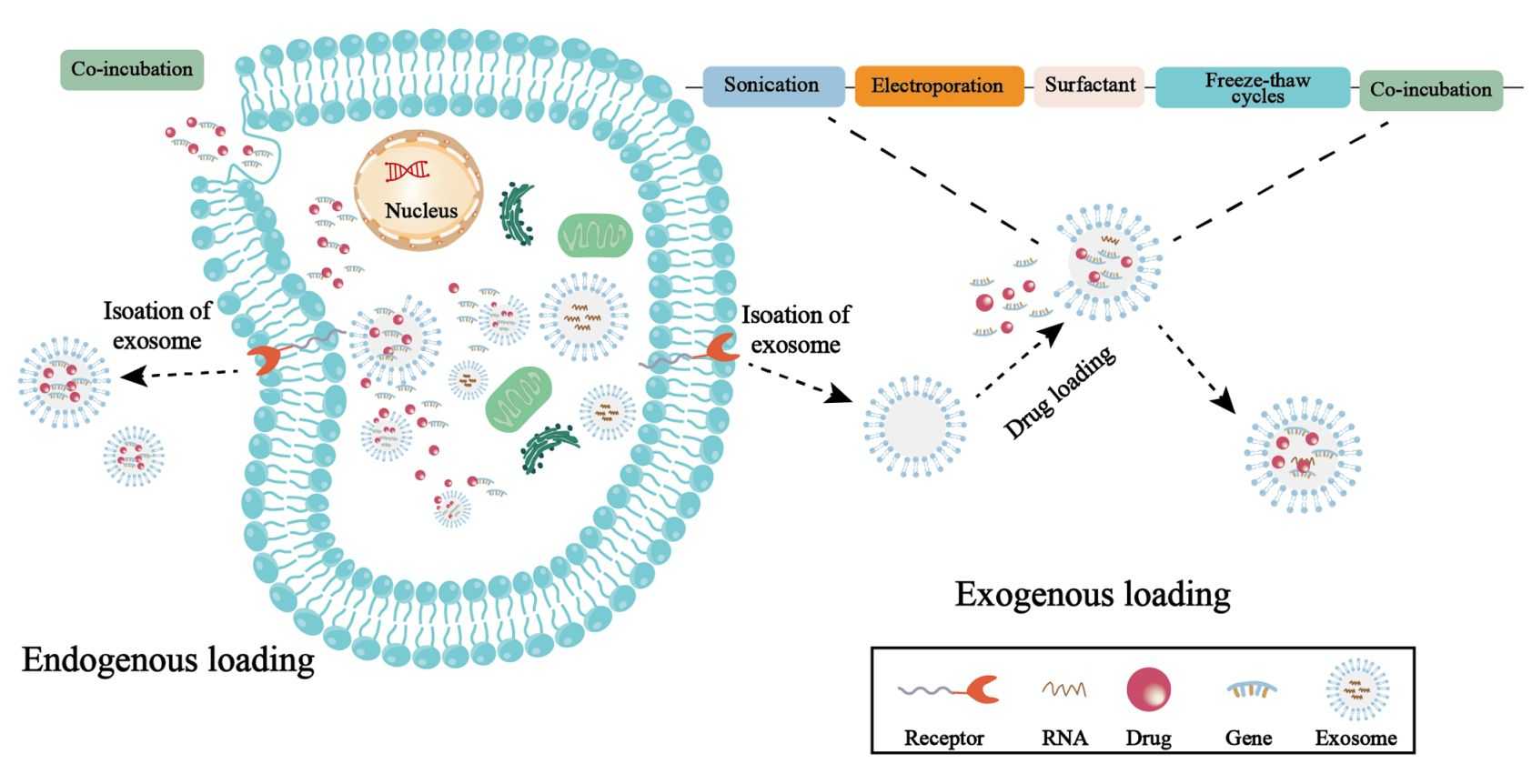 Figure 1. Endogenous loading and exogenous loading of material engineering exosomes. (Huang L, et al., 2023)
Figure 1. Endogenous loading and exogenous loading of material engineering exosomes. (Huang L, et al., 2023)
Advantages of Exosomes for Drug Delivery
Many new drug candidates (e.g., proteins and nucleic acids) are highly unstable in the body environment, posing significant challenges to the effectiveness of therapeutic outcomes. Exosomes allow the delivery of these biomolecules as a "natural delivery system". Due to the small size of exosomes and the fact that they are themselves cellular products, the delivery of drugs via exosomes avoids phagocytosis or degradation by macrophages, and allows for prolonged circulation in the body to maintain effectiveness. Of these, the ability to cross the blood-brain barrier to deliver specific drugs to the central nervous system is a significant advantage of exosomal drug delivery.
What is Extrusion Technology?
Extrusion technology involves extruding isolated exosomes with therapeutic drugs through membrane filters with a pore size of 100-400nm. The exosome-drug mixture is repeatedly passed through the membrane pores under controlled pressure. This process facilitates the physical encapsulation of the therapeutic drug within the exosome. Research has found that extrusion helps to homogenize the size distribution of exosomes. In addition, the extrusion technique can efficiently incorporate drugs into exosomes with significantly higher efficiency in enzyme incorporation than incubation, freeze-thawing, and incubation with saponins. However, repeated extrusion results in exosomes and cargoes that may be damaged by excessive shear stress. In some cases, membrane composition or orientation may not be affected, as evidenced by characterizing exosomes (maintenance of zeta potential and membrane protein profile) after extrusion.
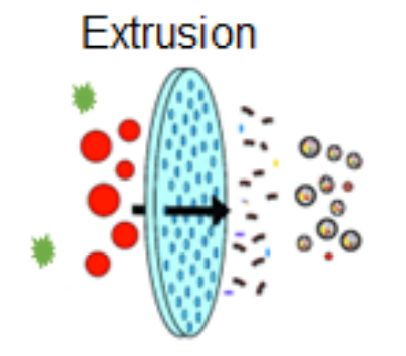 Figure 2. Schematic of exosome drug loading based on extrusion. (Poinsot V, et al., 2024)
Figure 2. Schematic of exosome drug loading based on extrusion. (Poinsot V, et al., 2024)
Application Cases of Exosome Drug Delivery by Extrusion
Effective drug loading is a challenge that limits the clinical translation of exosome-based drug delivery. Researchers developed a facile magnetic extrusion method for the preparation of endosome-derived vesicles, also known as exosome mimics (EMs), with the same biological origin and morphology, composition, and biological functions as natural exosomes. In addition, this method promotes the active loading of chemical drugs such as adriamycin into exosomes. The developed engineered exosomes exhibited drug delivery properties comparable to those of natural exosomes and effectively inhibited tumor growth by delivering adriamycin in an in situ breast tumor model.
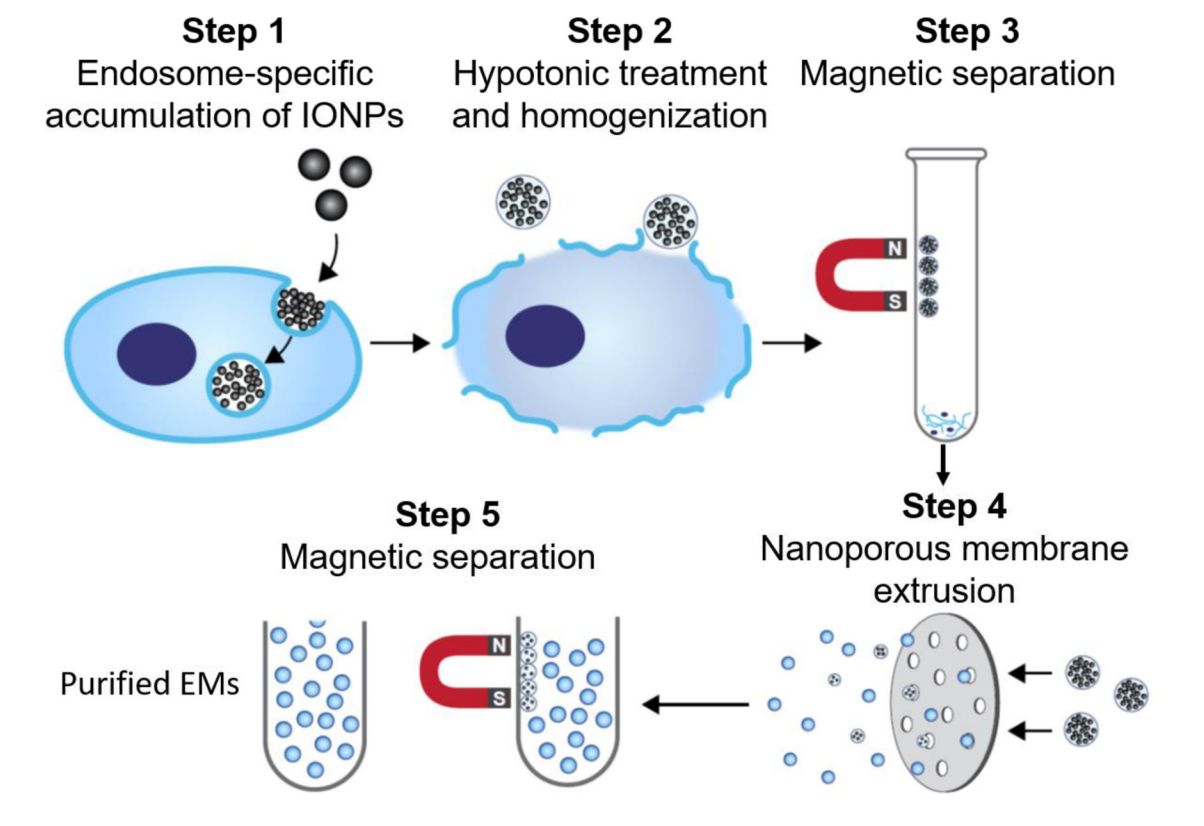 Figure 3. Schematic illustration of the magnetic extrusion method. (Guo P, et al., 2021)
Figure 3. Schematic illustration of the magnetic extrusion method. (Guo P, et al., 2021)
Angiogenesis is critical in diabetic wound healing. However, there is no effective strategy to target endothelial cells (EC) to promote diabetic wound healing. The research found that dagliflozin (DA) promotes neovascularization in diabetic mice through HIF-1α-mediated enhancement of angiogenesis. Researchers prepared biomimetic nanovesicles (NV) from EC by extrusion, which served as exosome mimics for targeted delivery of DA. These DA-loaded induced pluripotent stem cells (iPSC)-EC NV could promote angiogenesis and diabetic wound healing through the HIF-1α/VEGFA pathway.
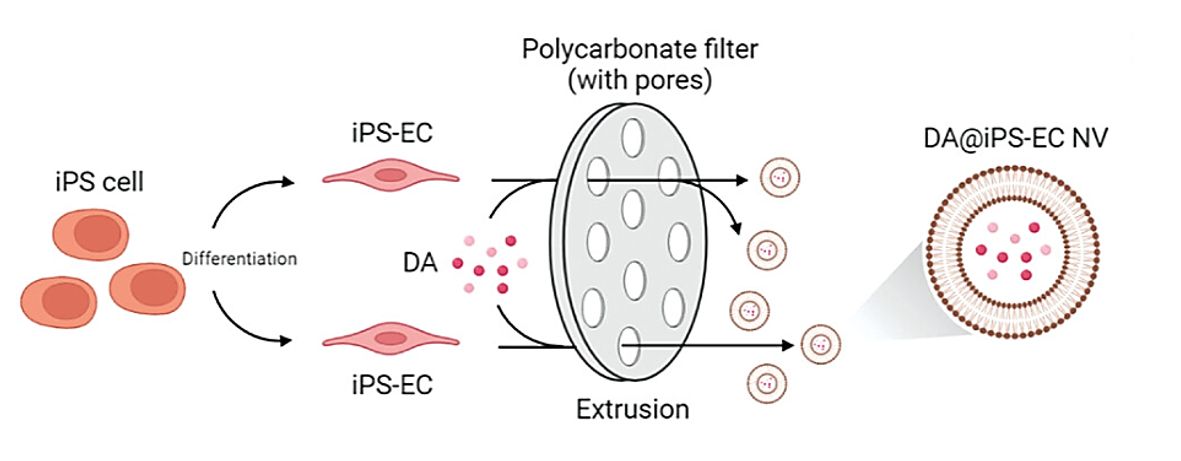 Figure 4. Schematic diagram of the extrusion method to obtain NVs to mimic exosomes. (Zhang W, et al., 2023)
Figure 4. Schematic diagram of the extrusion method to obtain NVs to mimic exosomes. (Zhang W, et al., 2023)
Our Products
Exosomes are naturally occurring nanovesicles that have attracted much attention as drug delivery vehicles over the past few years. Efficient drug delivery is critical for the clinical application of exosomes. Therefore, we provide extrusion-based exosome drug delivery services to help clients explore the possibility that exosomes may be a valuable tool in the future for the therapy of cancer and many chronic diseases.
In addition, as a leader in the field of exosome research, we offer a range of high-quality exosome products for exploring the therapeutic and drug transport functions of exosomes.
| Cat No. | Product Name | Source |
| Exo-CH08 | HQExo™ Exosome-BPH-1 | Exosome derived from human benign prostatic hyperplasia-1 (BPH-1 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH20 | HQExo™ Exosome-COLO205 | Exosome derived from human colon carcinoma (COLO205 cell line) |
| Exo-HDBF-02 | HQExo™ Exosome-SDH-Asthma plasma | Exosome derived from Single Donor Human Asthma plasma |
| Exo-HDBF-05 | HQExo™ Exosome-SDH-Benign Organ Tumor plasma | Exosome derived from Single Donor Human Benign Organ Tumor plasma |
| Exo-F488-BLCL | HQExo™ Exosome-488-BLCL | Fluorescent labeled exosome derived from EBV transformed lymphoblastoid B cells (BLCL21 cell line) |
| Exo-F488-COLO | HQExo™ Exosome-488-COLO | Fluorescent labeled exosome derived from human colon carcinoma (COLO1 cell line) |
| Exo-IC06 | HQExo™ Exosome-NK | Exosome derived from human NK cell line |
| Exo-IC03 | HQExo™ Exosome-Jurkat E6-1 | Exosome derived from human T lymphocyte cell line (Jurkat E6-1) |
| Exo-PDELN05 | HQExo™ Exosome-Seaberries | Exosome derived from Seaberries |
| PNE-FLB23 | PNExo™ Exosome-Balsam | Exosome derived from Balsam |
| Explore All Exosomes Products | ||
Creative Biostructure offers a one-stop shop for exosome services, including exosome isolation, characterization, labeling, tracking, targeting, and functional analysis. If you are interested in our services and products, please feel free to contact us for more details.
References
- Huang L, et al. Research Advances of Engineered Exosomes as Drug Delivery Carrier. ACS Omega. 2023. 8(46): 43374-43387.
- Poinsot V, et al. Engineered and Mimicked Extracellular Nanovesicles for Therapeutic Delivery. Nanomaterials. 2024. 14(7): 639.
- Guo P, et al. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv Funct Mater. 2021. 31(44): 2008326.
- Zhang W, et al. Dapagliflozin-Loaded Exosome Mimetics Facilitate Diabetic Wound Healing by HIF-1α-Mediated Enhancement of Angiogenesis. Adv Healthc Mater. 2023. 12(7): e2202751.
- Zeng H, et al. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells. 2023. 12(10): 1416.