Hydrophobic Interaction Chromatography (HIC)
Hydrophobic Interaction Chromatography (HIC) is a chromatographic technique designed to separate biomolecules based on their hydrophobic properties. It is particularly useful for the purification and analysis of proteins, peptides and other biomolecules. HIC plays an important role in structural biology, providing insight into the hydrophobic interactions that govern protein folding and stability.
Basic Principles of HIC
HIC separates molecules based on their hydrophobic properties. The stationary phase in HIC columns is typically composed of hydrophobic materials, such as alkyl chains (e.g., octyl or phenyl groups) attached to a solid support. During the chromatographic process, proteins or other biomolecules in the sample interact with these hydrophobic groups based on their own hydrophobicity.
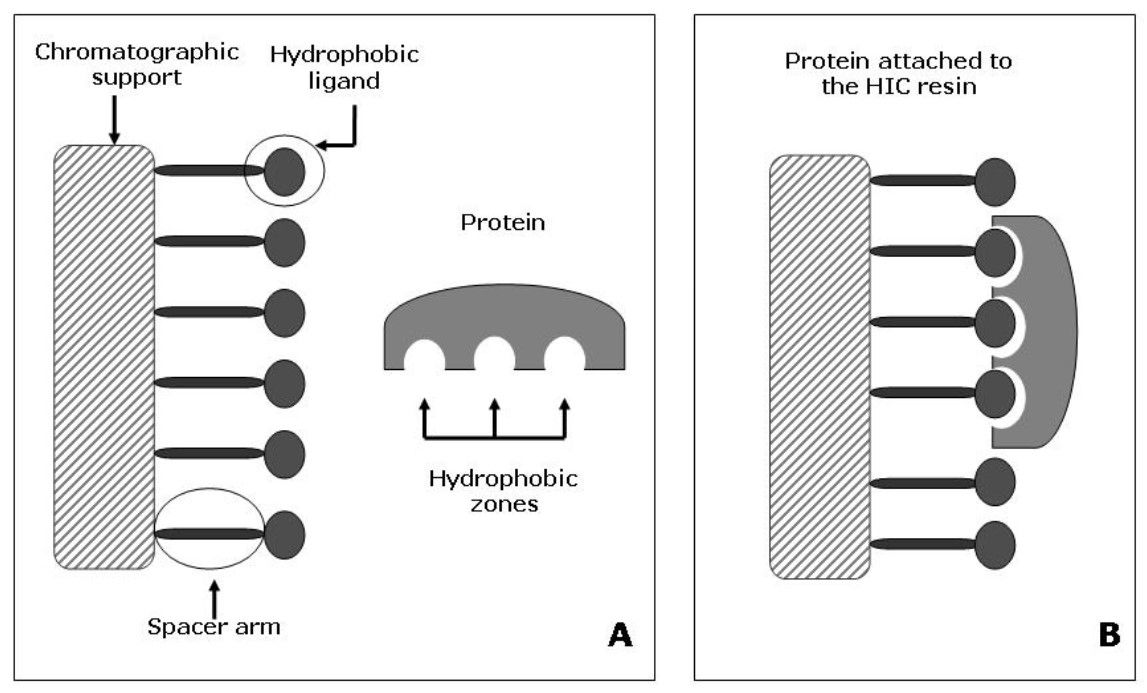 Figure 1. Protein retention mechanism in HIC. (A) The basic structure of a HIC resin is depicted, and a protein is schematized highlighting the hydrophobic zones on the protein surface. (B) The protein gets in contact with the hydrophobic ligands of the resin, suffering a spatial reorientation. The hydrophobic ligands of the matrix interact with the exposed hydrophobic zones of the protein, and thus the protein is reversibly attached to the resin (Mahn, 2012).
Figure 1. Protein retention mechanism in HIC. (A) The basic structure of a HIC resin is depicted, and a protein is schematized highlighting the hydrophobic zones on the protein surface. (B) The protein gets in contact with the hydrophobic ligands of the resin, suffering a spatial reorientation. The hydrophobic ligands of the matrix interact with the exposed hydrophobic zones of the protein, and thus the protein is reversibly attached to the resin (Mahn, 2012).
Workflow of HIC
Sample Loading: The sample is applied to the HIC column under high salt conditions. High salt concentrations promote hydrophobic interactions by decreasing the solubility of proteins in the aqueous phase, thereby encouraging their binding to the hydrophobic stationary phase.
Binding: Hydrophobic molecules in the sample bind to the hydrophobic groups on the stationary phase due to their nonpolar nature. The strength of this interaction depends on the extent of hydrophobicity of the molecule.
Washing: After loading, the column is washed with a buffer containing high salt to remove unbound or weakly bound impurities. This step ensures that only strongly hydrophobic proteins remain bound to the column.
Elution: To elute the bound proteins, the salt concentration is gradually reduced or a more polar solvent is introduced. This decrease in hydrophobic interaction strength allows the proteins to desorb from the stationary phase and be eluted from the column. Proteins with less hydrophobicity elute earlier, while those with higher hydrophobicity elute later.
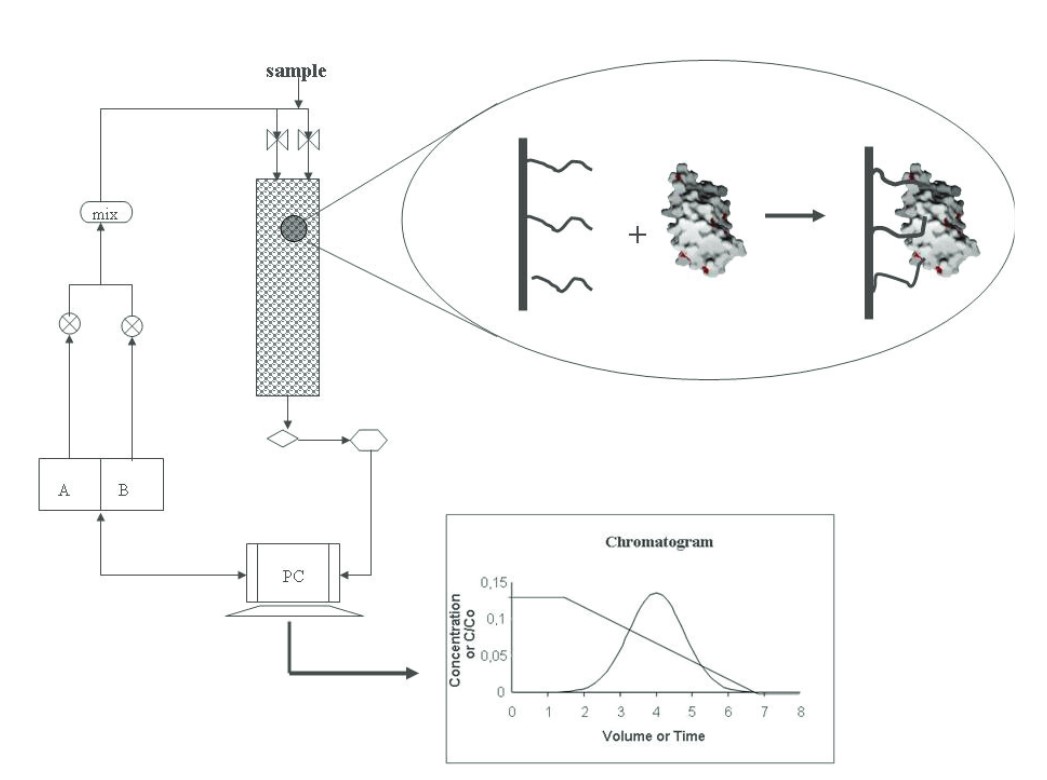 Figure 2. Schematic representation of the HIC process. The HIC process consists of injecting a protein sample in a hydrophobic column under high salt concentration conditions such that hydrophobic interaction between the protein and the resin is promoted. Elution is achieved by decreasing the ionic strength in the mobile phase, building a decreasing salt gradient. In a microscopic level, the hydrophobic patches on the protein surface interact with the hydrophobic ligands of the resin, being reversibly attached to it. The protein concentration in the outlet is recorded as a function of time, and then a chromatogram is obtained (Mahn, 2012).
Figure 2. Schematic representation of the HIC process. The HIC process consists of injecting a protein sample in a hydrophobic column under high salt concentration conditions such that hydrophobic interaction between the protein and the resin is promoted. Elution is achieved by decreasing the ionic strength in the mobile phase, building a decreasing salt gradient. In a microscopic level, the hydrophobic patches on the protein surface interact with the hydrophobic ligands of the resin, being reversibly attached to it. The protein concentration in the outlet is recorded as a function of time, and then a chromatogram is obtained (Mahn, 2012).
Advantages of HIC
HIC offers several significant advantages for structural biology. These are summarized in the table below:
Table 1. Advantages of HIC technique.
| High Resolution | HIC provides high-resolution separation of proteins and other biomolecules based on their hydrophobicity. This allows for the effective isolation of proteins from complex mixtures, which is essential for structural studies. |
| Mild Conditions | The conditions used in HIC (such as high salt concentrations) are relatively mild compared to other chromatographic techniques. This helps in preserving the biological activity and integrity of sensitive proteins, making it suitable for applications involving delicate biomolecules. |
| Simplicity and Versatility | HIC is relatively straightforward to perform and does not require complex sample preparation. Its versatility allows it to be used for various applications, including protein purification, characterization, and engineering. |
| Scalability | HIC can be scaled from analytical to preparative applications, making it suitable for both small-scale studies and large-scale protein production. |
Limitations of HIC
However, HIC also has limitations:
Table 2. Limitations of HIC technique.
| Limited Resolution for Low Hydrophobicity Proteins | Proteins with very low hydrophobicity may not interact strongly with the hydrophobic stationary phase, leading to poor resolution or even ineffective separation. This limitation can be addressed by optimizing the conditions or using alternative chromatographic methods. |
| Salt Effects | The high salt concentrations required for binding can sometimes lead to issues with sample solubility or stability. Moreover, the salt concentration needs to be carefully controlled to avoid protein precipitation or other undesirable effects. |
| Specificity | HIC is less specific than some other chromatographic techniques, such as affinity chromatography, which targets specific interactions. As a result, it may not provide the same level of resolution for certain applications that require precise separation based on specific molecular properties. |
| Column Fouling | Hydrophobic interactions can sometimes lead to non-specific binding or fouling of the column, which can affect its performance and lifespan. Regular maintenance and careful handling are required to mitigate this issue. |
Applications of HIC
Protein Purification: HIC is extensively used for the purification of proteins, particularly when dealing with complex mixtures. The technique efficiently separates proteins based on their hydrophobicity, allowing for the isolation of target proteins from contaminants. This is crucial for obtaining pure samples for further structural and functional studies.
Characterizing Protein-Ligand Interactions: Hydrophobic interactions play a significant role in protein-ligand binding. HIC can be employed to analyze these interactions by studying how different ligands affect the hydrophobic interactions of the protein. This helps in understanding the binding affinity and the nature of the interaction.
Studying Protein Aggregation and Folding: Protein aggregation and folding are often influenced by hydrophobic interactions. HIC can be used to investigate these phenomena by analyzing how proteins with different folding states interact with the hydrophobic stationary phase. This can provide insights into protein stability and aggregation processes.
Protein Engineering and Design: In protein engineering, altering the hydrophobic properties of proteins can be a strategy to modify their function or stability. HIC is used to screen engineered proteins for changes in hydrophobicity, helping in the selection of variants with desirable properties.
In conclusion, Hydrophobic Interaction Chromatography (HIC) is a valuable technique in the field of chromatography, particularly for the separation and analysis of biomolecules based on their hydrophobic properties. Its principles are grounded in the interaction of hydrophobic groups on the stationary phase with hydrophobic regions of proteins and other molecules. HIC offers several advantages, including high resolution, mild conditions, and versatility, making it suitable for various applications in structural biology, such as protein purification, characterization, and protein engineering. However, it also has limitations, including challenges with low hydrophobicity proteins, salt effects, specificity issues, and potential column fouling. Understanding these aspects allows researchers to effectively utilize HIC in their studies and to complement it with other chromatographic techniques as needed.
At Creative Biostructure, we offer Hydrophobic Interaction Chromatography (HIC) services designed for advanced structural biology applications. Our HIC technology enables the efficient separation of proteins, peptides, and other biomolecules based on hydrophobicity, delivering precise and reliable results. With cutting-edge equipment and expert support, we are committed to enhancing your research. Reach out to us today to discover how our HIC services can drive your biomolecular analysis forward.
References
- Mahn, A. (2012). Hydrophobic interaction chromatography: Fundamentals and applications in biomedical engineering.
- Roettger, B. F., & Ladisch, M. R. (1989). Hydrophobic interaction chromatography. Biotechnology Advances, 7(1), 15–29.