Structural Research of Translocator Protein (18 kDA) TSPO
The translocator protein (18 kDA) TSPO is found predominantly on the outer mitochondrial membrane and was originally described as a peripheral benzodiazepine receptor (PBR). In humans, TSPO belongs to the tryptophan-rich sensory protein family involved in essential mitochondrial-based physiological processes such as cholesterol transport, steroidogenesis, cellular bioenergetics, apoptosis, and mitochondrial respiration. In recent years, TSPO has become the focus of intensive research in the biomedical and pharmaceutical industries due to its involvement in many disease-related processes. The resolution of TSPO structural information also provides a platform for structure-based drug development.
Function of TSPO in steroid hormone biosynthesis
Indeed, TSPO is widely distributed in the brain and plays a major role in neuroinflammation. Induction of neurosteroid formation in the brain by TSPO pharmacological ligands has been used to alleviate symptoms of neuropsychiatric disorders. Extensive research on the biochemistry and pharmacology of TSPO has shown that it specifically binds chemicals such as benzodiazepines, isoquinoline carboxamides, and indole acetamides. Its structural information reveals that drug-ligand binding promotes cholesterol movement in TSPO.
Progress in structural research of TSPO
Structural determination of TSPO is essential to reveal its function. Recent research on TSPO, which is highly abundant in the outer mitochondrial membrane (OMM) of steroidogenic cells, has provided detailed information on the atomic structure of TSPO through structural studies by NMR and crystallography. The structure shows that TSPO is an evolutionarily highly conserved OMM protein that is dimeric and has five transmembrane structural domains (TMs). The transmembrane regions TM1 and TM2 are connected by a long loop (LP1). A cholesterol-binding CRAC structural domain (L/ V-X (1-5) -Y-X (1-5)-R/K) is identified at the carboxy terminus of TM5 of TSPO.
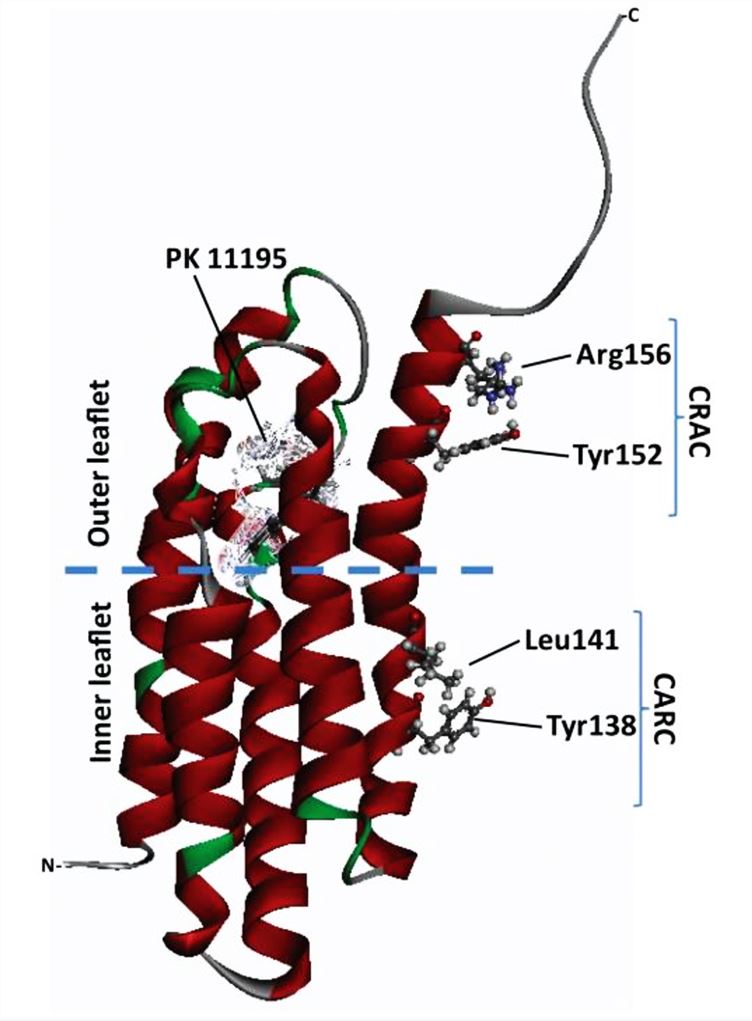 Figure 1. Overview of TSPO structures. (Papadopoulos V, et al., 2018)
Figure 1. Overview of TSPO structures. (Papadopoulos V, et al., 2018)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Translocator protein 18 kDa (TSPO) | Cereibacter sphaeroides | X-ray diffraction | 2.5 Å | 4UC3 |
| Translocator protein 18kDa (TSPO) (A139T mutant) in P212121 space group | Cereibacter sphaeroides | X-ray diffraction | 2.4 Å | 4UC2 |
| Translocator protein 18kDa (TSPO) (A139T Mutant) in C2 space group | Cereibacter sphaeroides | X-ray diffraction | 1.8 Å | 4UC1 |
| Apo dimer of BcTSPO | Bacillus cereus ATCC 14579 | X-ray diffraction | 4.1 Å | 4RYJ |
| BcTSPO/PK11195 complex | Bacillus cereus ATCC 14579 | X-ray diffraction | 3.49 Å | 4RYI |
| BcTSPO Iodo Type1 monomer | Bacillus cereus ATCC 14579 | X-ray diffraction | 2.8 Å | 4RYM |
| BcTSPO, type 2 | Bacillus cereus ATCC 14579 | X-ray diffraction | 1.7 Å | 4RYQ |
| BcTSPO, type1 monomer | Bacillus cereus ATCC 14579 | X-ray diffraction | 2.01 Å | 4RYN |
| Native translocator protein 18kDa (TSPO) (A139T Mutant) in C2 space group | Cereibacter sphaeroides | X-ray diffraction | 2.4 Å | 5DUO |
| Mitochondrial translocator protein (TSPO) in complex with its high-affinity ligand PK11195 | Mus musculus | SOLUTION NMR | / | 2MGY |
| A147T variant of the mitochondrial translocator protein (TSPO) in complex with pk11195 | Mus musculus | SOLUTION NMR | / | 2N02 |
| RsTSPO A138F with one Heme bound | Cereibacter sphaeroides | X-ray diffraction | 2.1 Å | 8E7X |
| RsTSPO A138F with two heme bound | Cereibacter sphaeroides | X-ray diffraction | 2.3 Å | 8E7Y |
| RsTSPO A139T with Heme | Cereibacter sphaeroides | X-ray diffraction | 2.1 Å | 8E7W |
| RsTSPO mutant -A138F | Cereibacter sphaeroides | X-ray diffraction | 2.6 Å | 8E7Z |
| BcTSPO type II high-resolution monomer | Bacillus cereus ATCC 14579 | X-ray diffraction | 1.6 Å | 4RYO |
| BcTSPO, type 2 with DMSO | Bacillus cereus ATCC 14579 | X-ray diffraction | 1.704 Å | 4RYR |
Table 1. Structural research of the translocator protein (18 kDA) TSPO.
Creative Biostructure is a preeminent company providing innovative structural analysis services, leveraging cutting-edge structural biology techniques to research the structure and function of biomolecules. Our scientists have extensive experience in X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy. This enables us to provide detailed structural analysis services of the translocator protein (18 kDA) TSPO, helping clients to reveal the potential of TSPO as a biomarker and drug target.
With our unrivaled expertise in structural biology, we can provide accurate and detailed insights into the structure and function of membrane proteins. If you would like to learn more about our services or would like to discuss your project with one of our expert scientists, please do not hesitate to contact us. Our team of distinguished scientists is always on hand to provide the best solution for your project.
References
- Papadopoulos V, et al. Translocator protein (18 kDa): an update on its function in steroidogenesis. J Neuroendocrinol. 2018. 30(2): 10.1111/jne.12500.
- Li F, Liu J, et al. Evolving understanding of translocator protein 18 kDa (TSPO). Pharmacol Res. 2015.99: 404-409.
- Fan J, et al. Structural and functional evolution of the translocator protein (18 kDa). Curr Mol Med. 2012. 12(4): 369-386.
