Mesenchymal Stem Cell-derived Exosomes (MSC-Exos) in Immunomodulation
Mesenchymal stem cells (MSCs) are a class of multipotent stem cells initially found in bone marrow and later shown to be widespread in tissues such as adipose, umbilical cord, dental pulp, and hair follicles. MSC-derived exosomes (MSC-Exos) carry biologically active substances from donor cells and thus may have applications similar to those of MSCs (regenerative, anti-inflammatory, immunomodulatory, etc.).
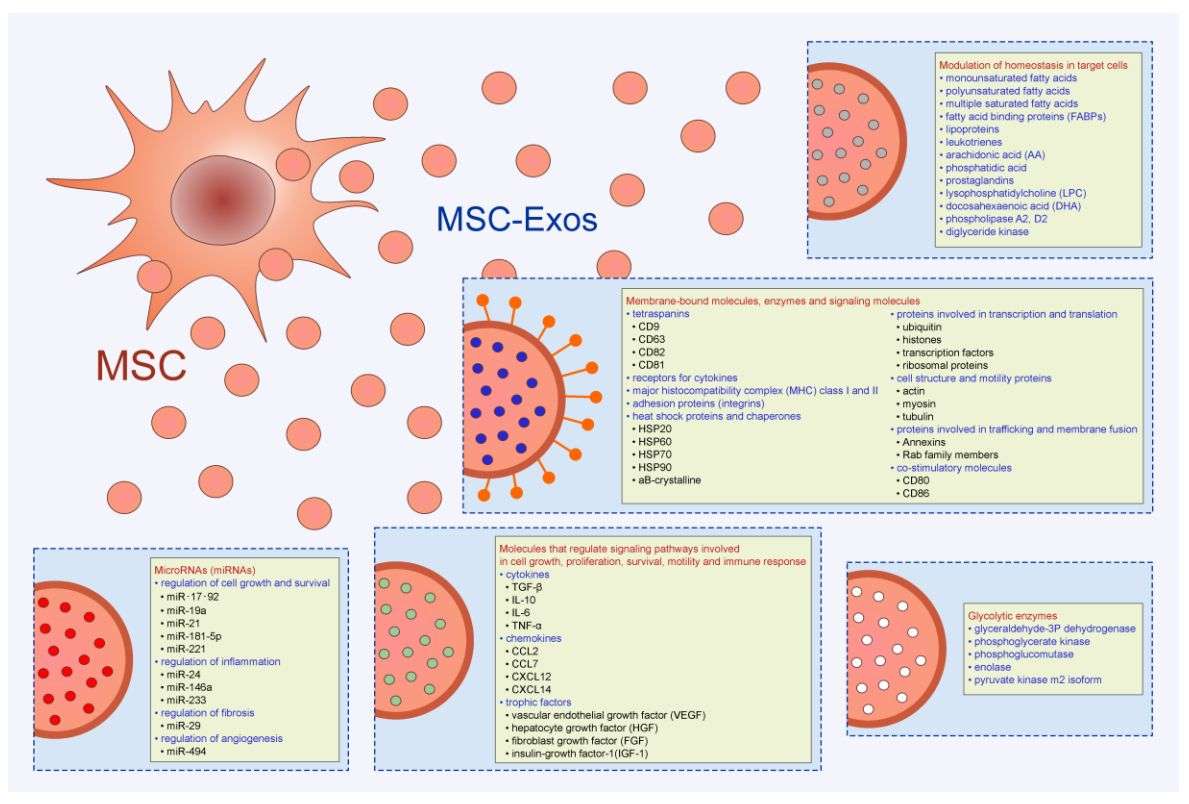 Figure 1. Composition and role of mesenchymal stem cell exosomes (MSC-Exos). (Harrell CR, et al., 2020)
Figure 1. Composition and role of mesenchymal stem cell exosomes (MSC-Exos). (Harrell CR, et al., 2020)
Studies have shown that MSC-Exos can play an immunoregulatory role by influencing multiple immune cells, and show good application prospects in autoimmune diseases. In recent years, more and more scholars have proposed to use MSC-Exos to replace MSCs to play the role of immunoregulation, which can well avoid the risk of cell transplantation, and MSC-Exos have lower immunogenicity and stronger penetration ability.
Biological Properties of MSC-Exos
- Bioactive Molecules
MSC-Exos are enriched with a variety of bioactive molecules such as mRNAs, miRNAs, and proteins, which are highly biologically active signaling molecules involved in a variety of biological processes.
- Stability and Transport
Exosomes are stable and can be used as effective drug carriers and delivered to target tissues through multiple pathways in vivo. This property makes exosomes have great potential for biomedical applications.
- Immunomodulation
MSC-Exos have immunomodulatory effects, which can inhibit inflammation, promote tissue repair, and participate in the activation and differentiation of immune cells.
Immunoregulatory Effects of MSC-Exos
MSC-Exos exert immunomodulatory effects by inducing immune cells to differentiate into cells with a tolerogenic phenotype.
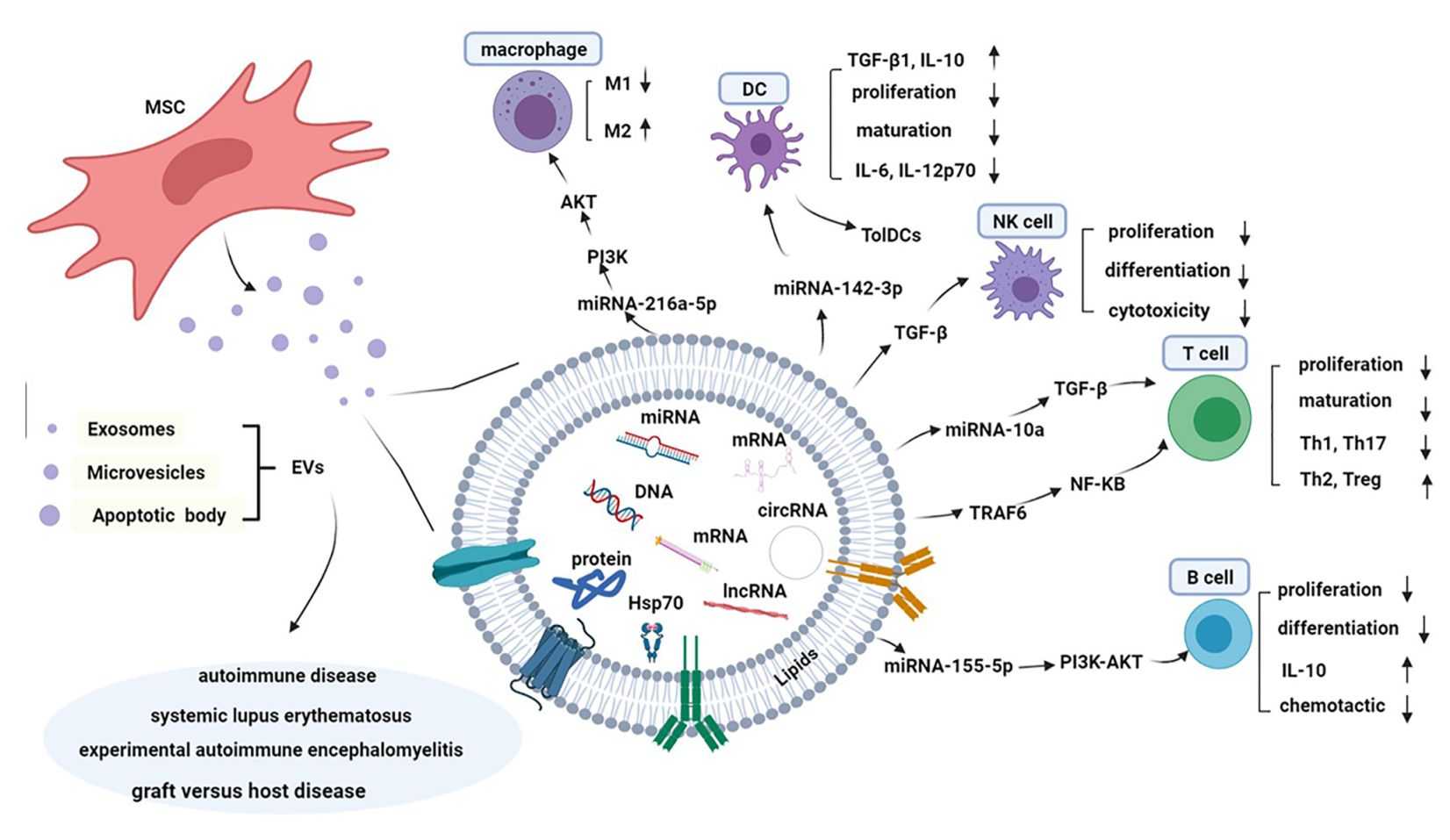 Figure 2. Composition and immune tolerance mechanism of MSC-Exos. (Yang C, et al., 2021)
Figure 2. Composition and immune tolerance mechanism of MSC-Exos. (Yang C, et al., 2021)
- MSC-Exos and Nonspecific Immunity
1. Macrophages
Macrophages are an important component of nonspecific immunity in the organism and are indispensable in damage repair, fighting pathogenic microorganisms, and maintaining the immune balance of the body. Studies have shown that human umbilical cord-derived MSC-Exo inhibits macrophage conversion to M1 type and down-regulates the expression of pro-inflammatory factors IL-1β and TNF-αwhile increasing the level of the anti-inflammatory factor IL-10 in an environment that induces macrophage conversion to the M1 type, thereby attenuating inflammatory responses.
2. Natural Killer Cells
Natural killer cells are important immune cells in the body, which not only can directly kill viruses or mutated cells without relying on T and B lymphocytes to exert their cytotoxic effects, but also participate in the body's immune surveillance and the occurrence of autoimmune diseases. It has been found that when natural killer cells are treated with human fetal liver MSCs and exosomes of their origin, the exosomes could perform functions similar to those of stem cells, including inhibition of proliferation, activation, and cytotoxicity of natural killer cells.
3. Dendritic Cells
Dendritic cells are the most potent antigen-presenting cells in the body and are at the dividing line between non-specific and acquired immunity. Studies have shown that dendritic cells play an extremely critical role in non-specific immunity, acquired immunity, and the maintenance of auto-tissue immune tolerance. MSC-Exos are found to affect the release of TGF-β, IL-10, and IL-6 from dendritic cells, which in turn affect the expression of dendritic cell co-stimulatory markers or the ability of dendritic cells to regulate lymphocyte proliferation.
- MSC-Exos and Acquired Immune
1. T-lymphocytes
T-lymphocytes originate from bone marrow pluripotent stem cells, develop and mature in the thymus, and are subsequently distributed to other tissues of the body via the bloodstream to function in cellular immunity. Studies have shown that MSC-Exo contains three negative feedback immunomodulatory molecules, PD-L1, TGF-β, and galectin-1. PD-L1 inhibits the immune response of T cells and promotes the proliferation of regulatory T cells, TGF-β induces the differentiation of monocytes to regulatory T cells, and galectin-1 induces apoptosis of already activated T cells.
2. B-lymphocytes
B-lymphocytes are the main effector cells of humoral immunity. After receiving antigen stimulation, mature B-lymphocytes differentiate into plasma cells under the joint action of helper T-cells and antigen-presenting cells, and the plasma cells can synthesize and secrete antibodies to play the role of humoral immunity. It is found that MSC-Exos could inhibit the proliferation of B lymphocytes and antibody secretion in a dose-dependent manner.
Medical Applications of MSC-Exos
MSC-Exos are a very promising cell-free therapeutic tool as a new strategy for the treatment of Alzheimer's disease, type I diabetes, cancer, chronic kidney disease, and more, says the study.
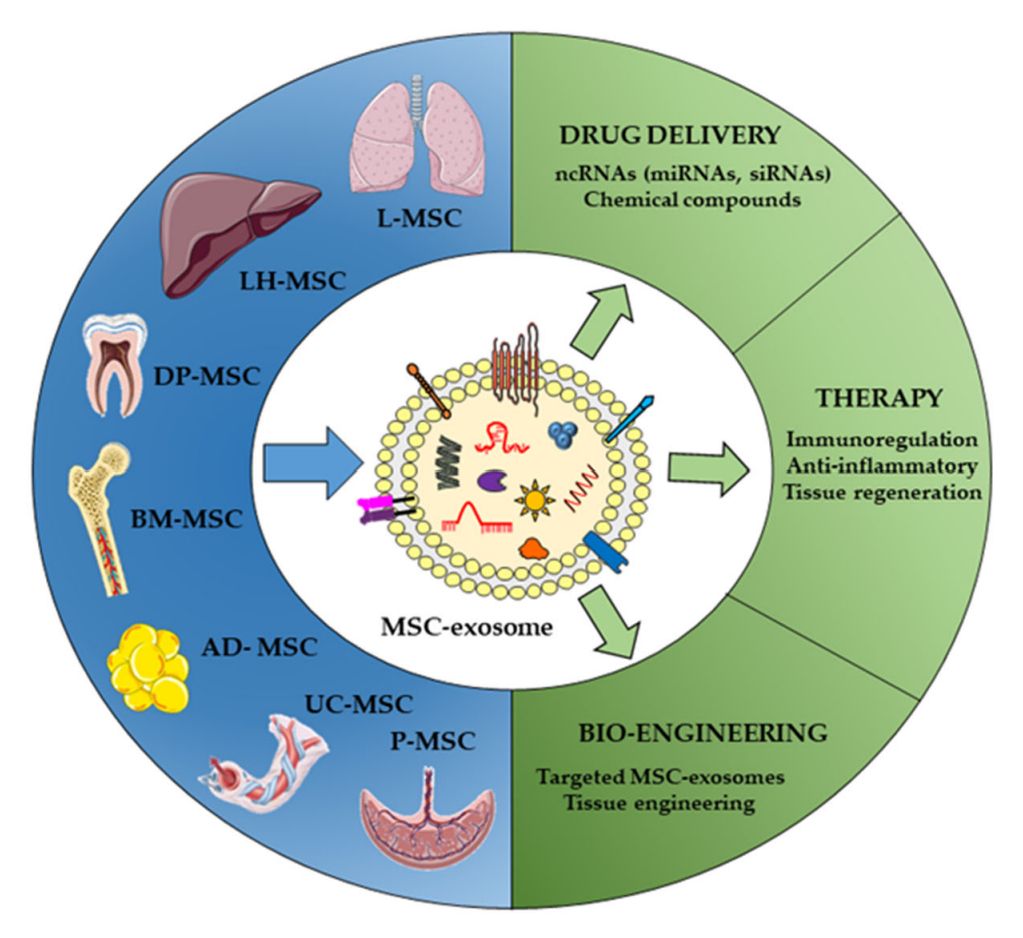 Figure 3. Origin and therapeutic purpose of MSC-Exos. (Martinez-Arroyo O, et al., 2022)
Figure 3. Origin and therapeutic purpose of MSC-Exos. (Martinez-Arroyo O, et al., 2022)
- MSC-Exos in the Treatment of Diabetes Mellitus
Current studies have introduced exosomes into the field of diabetes. Studies have shown that exosomes are involved in the occurrence and development of diabetes and its related complications, and they can not only be used as biological markers for early diagnosis and staging of diabetes, but also as targets for diabetes treatment. Some researchers obtained a large number of MSC-Exos loaded with endothelial-type nitric oxide synthase (eNOS) through genetic engineering and optogenetic techniques to observe their effects on the biological functions of fibroblasts and vascular endothelial cells. The results showed that MSC-Exos/eNOS significantly improved the biological functions of cells after high glucose treatment, promoted cell proliferation and migration, and simultaneously reduced oxidative stress-induced inflammatory factor expression and apoptosis.
- MSC-Exos in the Treatment of Cardiovascular Diseases
The cardioprotective mechanism of stem cell exosomes has become a hot research topic. More and more studies have confirmed that stem cell-derived exosomes have cardioprotective functions similar to those of stem cells, which can promote angiogenesis, reduce apoptosis, and minimize the damage caused by stress. Exosomes have better stability and are less likely to cause mutations in recipient cells as well as immune rejection, which is expected to be an effective treatment for cardiovascular diseases. MSC-Exos transplantation can significantly increase the survival rate of cardiomyocytes and can resist cardiomyocyte damage, promote neovascularization, and improve cardiac function. Other studies have also confirmed that MSC-derived exosome has anti-inflammatory, anti-apoptotic cardiomyocytes, and promotes cardiomyocyte regeneration.
- MSC-Exos in the Treatment of Chronic Kidney Disease
Chronic kidney disease (CKD) is a progressive and irreversible disease, and in a study, researchers used stem cell exosomes to improve the progression of CKD. Participants are randomized in a 1:1 ratio into a control group or an experimental group that received stem cell exosome therapy. The results of the experiment showed that those in the experimental group achieved significant improvements in everything from kidney function to indicators of kidney metabolic substance levels.
Our Services and Products
The excellent performance of exosomes in many types of research has led people to see their very broad application prospects. Exosomes may be effective in promoting gene therapy for cancer and other debilitating diseases.
As a leader in the field of exosome research and application, Creative Biostructure focuses on exploring the potential of MSC-Exos. With extensive expertise in exosome isolation, characterization, engineering, and analysis, we provide our clients with a reliable supply of high-quality MSC-Exos. By leveraging the natural ability of MSC-Exos to modulate immune responses, we develop cutting-edge solutions for various immune-mediated diseases. Please feel free to contact us for more information.
| Cat No. | Product Name | Source |
| Exo-SC02-1 | HQExo™ Exosome-PCS-500-012 | Exosome derived from human bone marrow-derived mesenchymal stem cell line (PCS-500-012) |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Exo-SC01 | HQExo™ Exosome-PCS-500-011 | Exosome derived from human pre-adipose derived mesenchymal stem cell (PCS-500-011) |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC04 | HQExo™ Exosome-MSC | Exosome derived from Xeno-Free Human Mesenchymal Stem/Stromal Cells and Media |
| Exo-C02 | CosExo™ Exosome-hMSC | Exosome derived from Human Umbilical Cord Mesenchymal Stem Cell |
| Explore All Exosome Isolated from Stem Cell Lines | ||
References
- Harrell CR, et al. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics. 2020. 12(5): 474.
- Yang C, et al. Immunomodulatory Effect of MSCs and MSCs-Derived Extracellular Vesicles in Systemic Lupus Erythematosus. Front Immunol. 2021. 12: 714832.
- Martinez-Arroyo O, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases. Pharmaceutics. 2022. 14(4): 733.