Structural Research of AbgT Family Transporters
Folic acid is involved in cell growth and division. In plants and most microorganisms, it must be synthesized de novo through the folate biosynthetic pathway, whereas mammals are unable to make folate themselves. Therefore, we can design and develop novel antimicrobial agents against pathogenic bacteria by targeting the folate biosynthetic pathway. Sulfonamides are widely used as antibiotics to treat infections caused by, for example, Pneumococcus and Neisseria meningitidis, which prevent pathogenic bacteria from using the metabolite p-aminobenzoic acid (PABA) for folate biosynthesis. However, pathogens quickly develop resistance to sulfonamides, and new targets in the folate synthesis pathway need to be sought.
Previous studies have shown that the AbgT family transporters promote the bacterial folate synthesis pathway by importing p-aminobenzoyl-glutamate for producing this vitamin, and the MtrF protein of this family of Neisseria gonorrhoeae acts as an antimicrobial resistance protein that is required for the high level of resistance to hydrophobic antimicrobial agents. Thus, the structural characterization of AbgT family transporters is of great significance for the development of new antibacterial drugs.
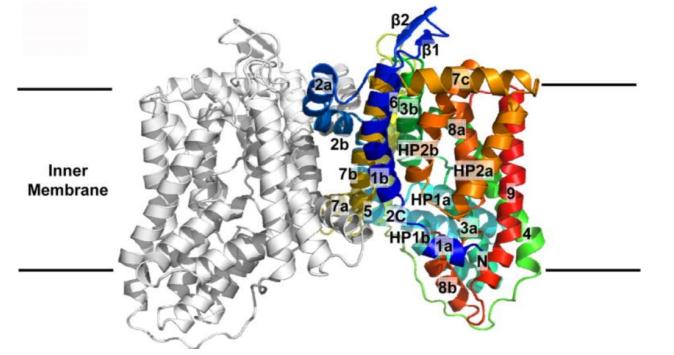 Figure 1. Transmembrane topology of YdaH. (Bolla J R, et al., 2015)
Figure 1. Transmembrane topology of YdaH. (Bolla J R, et al., 2015)
The YdaH transporter of Alcanivorax borkumensis was the first structure identified as a member of the AbgT family. The crystal structure of YdaH reveals a novel topology of dimers that is distinct from other families of transporters. YdaH protein is a bowl-shaped dimer with a solvent-filled basin extending from the cytoplasm to half of the transmembrane bilayer. Each subunit of YdaH contains nine transmembrane helices and two hairpins, indicating a possible pathway for substrate transport. And YdaH may also mediate bacterial resistance to sulfonamides by acting as an antibiotic efflux pump and removing sulfonamides from cells.
| Protein | Organism | Method | Resolution | PDB Entry ID |
| YdaH transporter (expressed in E. coli) | Alcanivorax borkumensis | X-ray diffraction | 2.96 Å | 4R0C |
Table 1. Structural Research of AbgT Family Transporters.
X-ray crystallography technique may yield high atomic resolution and is not limited by the molecular weight of the sample. Thus, it is suitable for water-soluble proteins, membrane proteins, as well as macromolecular complexes. When the X-ray crystallography technique is appropriately manipulated, it becomes a powerful tool for delivering reliable biological macromolecules' structural data. X-ray crystallography technique not only determines the active center's position and structure, but also helps understand how protein recognizes and binds ligand molecules at the atomic level.
Supported by years of experience, Creative Biostructure provides X-ray crystallography services in our state-of-the-art facilities and has developed an X-ray crystallography pipeline covering all technical stages, from gene synthesis to structure determination. We can offer various crystallization strategies, particularly for membrane protein crystallization. If you are interested in our services, please feel free to contact our experts, and they will offer professional and comprehensive solutions.
Reference
- Bolla J R, et al. Crystal structure of the Alcanivorax borkumensis YdaH transporter reveals an unusual topology. Nature Communications. 2015. 6(1): 1-10.