Structural Research of Epidermal Growth Factor Receptors
Epidermal Growth Factor Receptors (EGFRs) are a family of receptor tyrosine kinases that play a significant role in various cellular processes and are implicated in many types of cancer. The receptors exist in equilibrium between the monomeric and pre-dimerized states. Upon ligand binding, the receptors undergo strong lateral dimerization with proper assembly of their extracellular ligand-binding, single-span transmembrane, and cytoplasmic kinase domains. There are different modes of dimerization of the human EGFR transmembrane domain (TMD) in different membrane mimetics. Structural research of human epidermal growth factor receptors (HER or ErbB) has been of great interest in the scientific community due to its potential applications in developing new cancer therapies.
The structure of EGFRs is essential for understanding the signal propagation steps from the extracellular to cytoplasmic domains of the receptors. Different modes of dimerization of the human EGFR transmembrane domain (TMD) in different membrane mimetics have been proposed to support the cytoplasmic kinase domain configuration corresponding to the receptor active state. Recent studies using high-resolution NMR spectroscopy in various membrane-mimicking micellar environments have identified alternative HER2 transmembrane domain dimerization coupled with self-association of the membrane-embedded cytoplasmic juxtamembrane region. This dimerization mode appears to be capable of effectively inhibiting the receptor kinase activity.
Moreover, recent advancements in the structural research of EGFRs have also revealed the mutual influence of TMD and the lipid environment, as required for the proposed lipid-mediated activation mechanism. This interplay between concurrent processes in the lipid and protein during signal transduction has possible functional relevance.
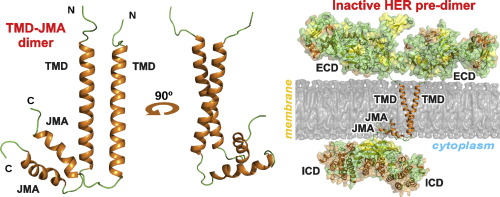 Figure 1. HER2-TMJMA in the micellar environment. (Bragin P E, et al., 2016)
Figure 1. HER2-TMJMA in the micellar environment. (Bragin P E, et al., 2016)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| ErbB2 transmembrane segment dimer (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2JWA |
| ErbB2 (HER2) transmembrane segment dimer with juxtamembrane region (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2N2A |
| ErbB1/ErbB2 transmembrane segment heterodimer (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2KS1 |
| ErbB1 transmembrane segment homodimer (expressed in E. coli) | Homo sapiens | Solution NMR | / | 5LV6 |
| ErbB3 transmembrane segment dimer (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2L9U |
| ErbB4 transmembrane segment dimer (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2LCX |
Table 1. Structural Research of Epidermal Growth Factor Receptors (EGFRs).
At Creative Biostructure, we offer a range of structural analysis services to help researchers better understand the molecular structure of proteins and other biomolecules. One of the key techniques we use is nuclear magnetic resonance (NMR) spectroscopy.
We provide a variety of NMR spectroscopy services, including but not limited to solution-state NMR, solid-state NMR, and dynamic nuclear polarization (DNP) NMR. We have state-of-the-art NMR equipment, including high-field NMR magnets, and a team of experienced NMR spectroscopists who can help you design and execute your experiments. Our NMR services are highly customizable, and we work closely with our clients to tailor our services to their specific research needs. We can provide NMR services for a wide range of applications, including protein-ligand interactions, protein-protein interactions, protein folding and dynamics, and many others.
In addition to NMR spectroscopy, we offer a range of other structural analysis services, such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and computational modeling. Our goal is to provide our clients with the most comprehensive suite of tools and expertise to help them solve complex structural biology problems and advance their research.
References
- Bragin P E, et al. HER2 transmembrane domain dimerization coupled with self-association of membrane-embedded cytoplasmic juxtamembrane regions. Journal of Molecular Biology. 2016, 428(1): 52-61.
- Bocharov E V, et al. The conformation of the epidermal growth factor receptor transmembrane domain dimer dynamically adapts to the local membrane environment. Biochemistry. 2017, 56(12): 1697-1705.