Transmission Electron Microscopy (TEM) for Exosome Characterization
Exosomes are extracellular vesicles secreted by most cells that play an essential role in intercellular communication by transporting a wide range of contents including proteins, mRNAs, miRNAs, and DNA. This cargo is closely associated with the pathogenesis of most human malignancies. Interest in exosomes is growing due to their extensive involvement in physiological and pathological processes, potential application as diagnostic and therapeutic tools, and biocarriers for exogenous molecules. Given their nanoscale size, transmission electron microscopy (TEM) has significantly contributed to assessing the presence and purity of exosomes and studying morphological characterization.
What is Transmission Electron Microscopy?
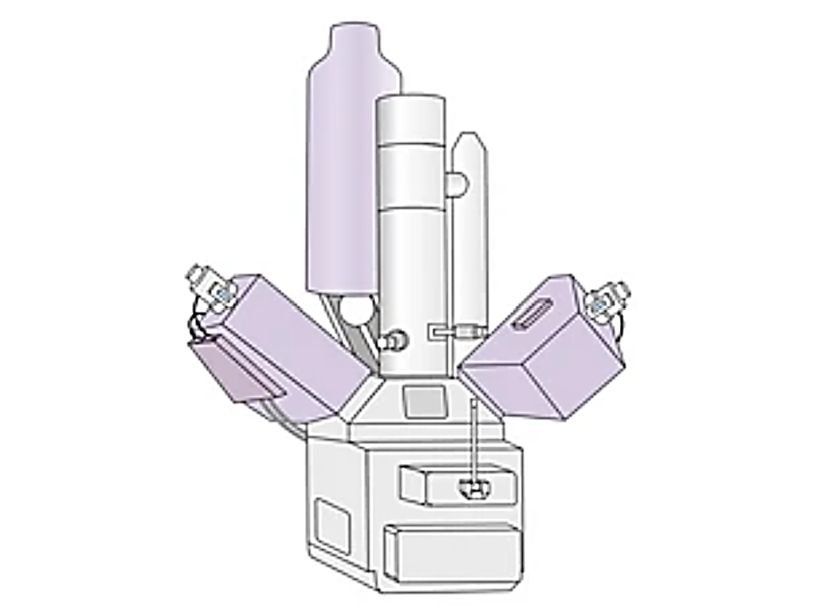
TEM is an analytical technique used to visualize the smallest structures in matter. Unlike optical microscopes, which rely on visible light, TEM can reveal amazing detail on the atomic scale by magnifying nanostructures up to 50 million times. Currently, TEM has a resolution of 0.2nm, and the images it generates can easily characterize the fine structure of the sample and describe physical and chemical properties.
Exosome Characterization Using Transmission Electron Microscopy
TEM is a common method used for exosome characterization. Samples prepared for TEM imaging are first fixed and then dehydrated. After dehydration, the samples must be embedded, sliced into nano-thin slices, and mounted on a carbon-coated grid for imaging. TEM uses an electron beam to irradiate the prepared sample; electrons can either transmit through the sample or be diffracted by the sample. A phosphor screen or charge-coupled device (CCD) will collect the transmitted electrons to obtain a bright-field image, which is often used for structural verification. At the same time, scattered electrons are collected to produce a dark field image, which shows the structure in higher contrast. Exosomes observed by TEM are often cupped due to dehydration during sample preparation, but can effectively reveal the internal structure of the exosome.
Principle of Transmission Electron Microscope
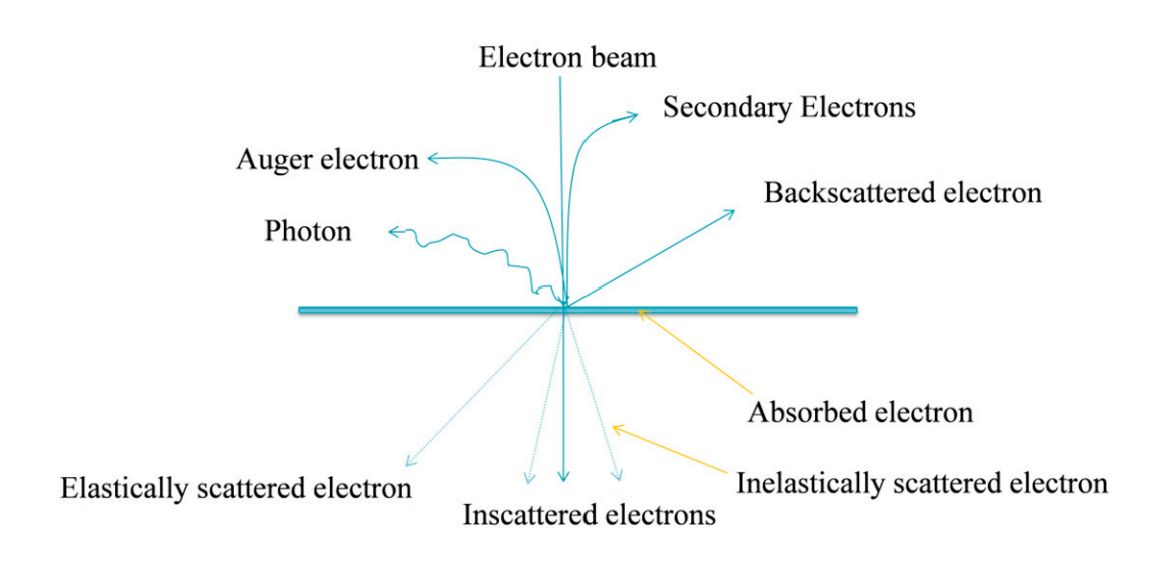 Figure 1. The basic principle of transmission electron microscopy (TEM). (Ahmed SS, et al., 2015)
Figure 1. The basic principle of transmission electron microscopy (TEM). (Ahmed SS, et al., 2015)
- The electron beam emitted by the electron gun, in the vacuum channel along the optical axis of the mirror body through the condenser mirror, through the condenser mirror will be converted into a beam of sharp, bright, and uniform spots, irradiated in the sample chamber on the sample.
- The electron beam through the sample carries the structural information inside the sample, and the amount of electrons transmitted through the sample is less in dense places and more in sparse places.
- After the objective lens convergence focusing and primary magnification, the electron beam enters the lower level of the intermediate lens and the first and second projection mirrors for integrated magnification imaging, and finally the magnified electron image is projected on the fluorescent screen in the observation room.
- The fluorescent screen converts the electronic image into a visible image for the user to observe.
How Does Transmission Electron Microscopy Image?
- Absorption image
When electrons are shot at a sample with high mass and density, the main phase-forming effect is scattering. The sample on the mass thickness of the place on the scattering angle of the electron is large, though the electron is less, the brightness of the image is darker.
- Diffraction image
After the electron beam is diffracted by the sample, the amplitude distribution of the diffracted wave at different positions of the sample corresponds to the different diffractive abilities of the various parts of the crystal in the sample. When there is a crystal defect, the diffractive ability of the defective part is different from that of the intact area, thus reflecting the distribution of the crystal defect.
- Phase image
When the sample is thin enough to be below 100Å, electrons can pass through the sample and the change in the amplitude of the wave can be neglected, and the imaging comes from the phase change.
What Components Makeup Transmission Electron Microscopy System?
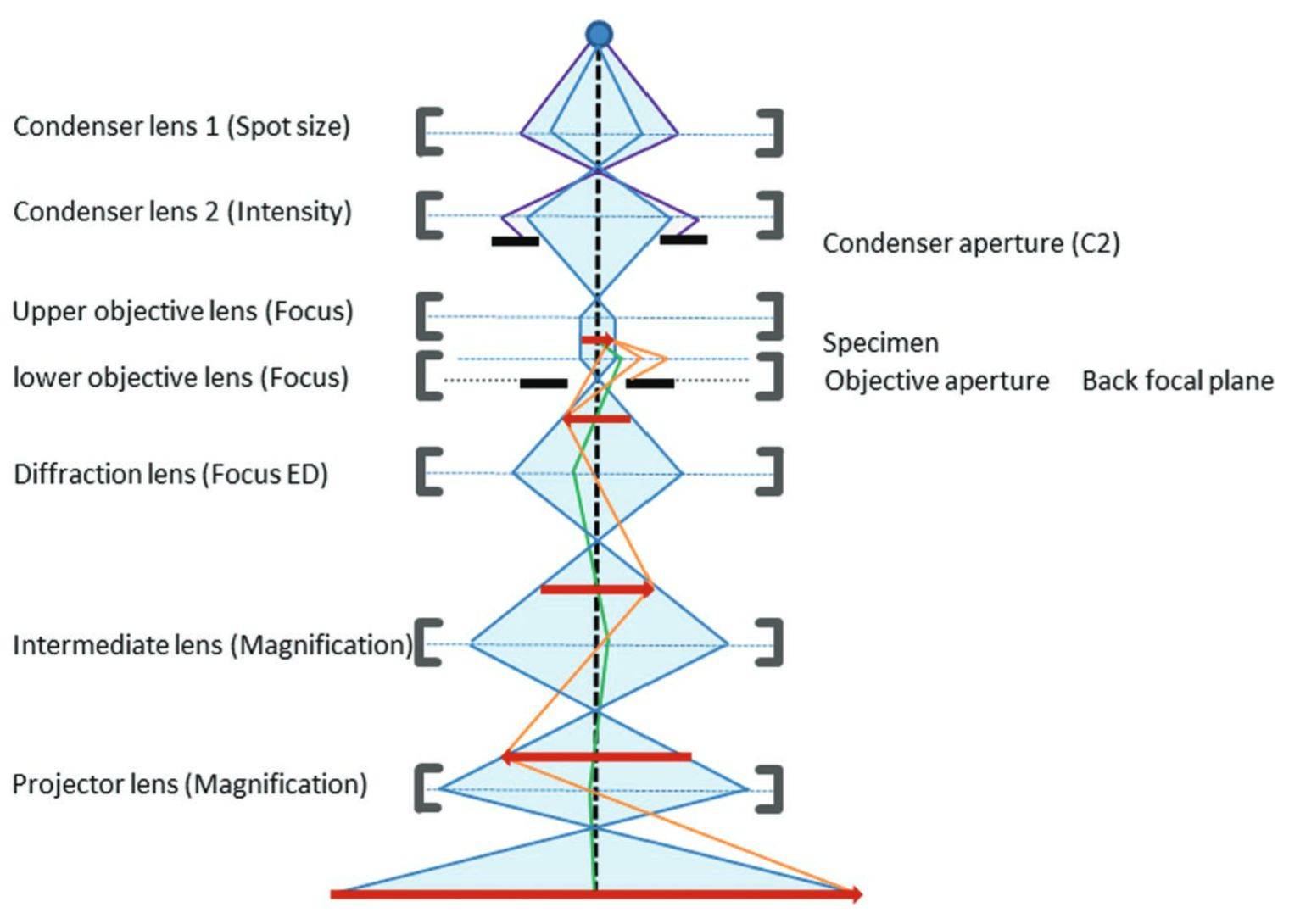 Figure 2. Schematic of the transmission electron microscope components. (Franken LE, et al., 2020)
Figure 2. Schematic of the transmission electron microscope components. (Franken LE, et al., 2020)
Electron gun - emits electrons and consists of a cathode, a grid, and an anode. The electrons emitted by the cathode tube form a ray beam through a small hole in the grid, which is accelerated by the anode voltage and then shot to the condenser mirror, which plays a role in accelerating and pressurizing the electron beam.
Concentrating mirror - the electron beam will be gathered, and can be used to control the intensity of illumination and aperture angle.
Sample room - placed to observe the sample, and equipped with a tilt table to change the angle of the specimen, as well as the assembly of heating, cooling, and other equipment.
Objective lens - for high magnification of short-distance lenses, the role is to magnify the electron image. The objective lens is to determine the resolution and imaging quality of the transmission electron microscope.
Intermediate mirror - a weak lens with variable magnification, which serves to magnify the electron image for the second time. By adjusting the current of the intermediate mirror, the image of the object or the electron diffraction pattern can be selected for magnification.
Transmission mirror - a high-magnification strong lens, used to magnify the intermediate image on the fluorescent screen after the image.
Two-stage vacuum pump - to vacuum the sample chamber.
Photographic device - used to record the image.
Preparing Specimens for Transmission Electron Microscopy Visualization
Specimens viewed under TEM must undergo special preparation techniques to visualize and create clear images. Samples should be approximately 20-100nm thick and 0.025-0.1nm in diameter. Thin samples allow interaction with electrons in the vacuum of space and can maintain their intrinsic structure. Clients are required to provide high-purity exosomes or provide samples for us to isolate exosomes. We have extensive experience in TEM specimen preparation and offer preparation programs for a wide range of products.
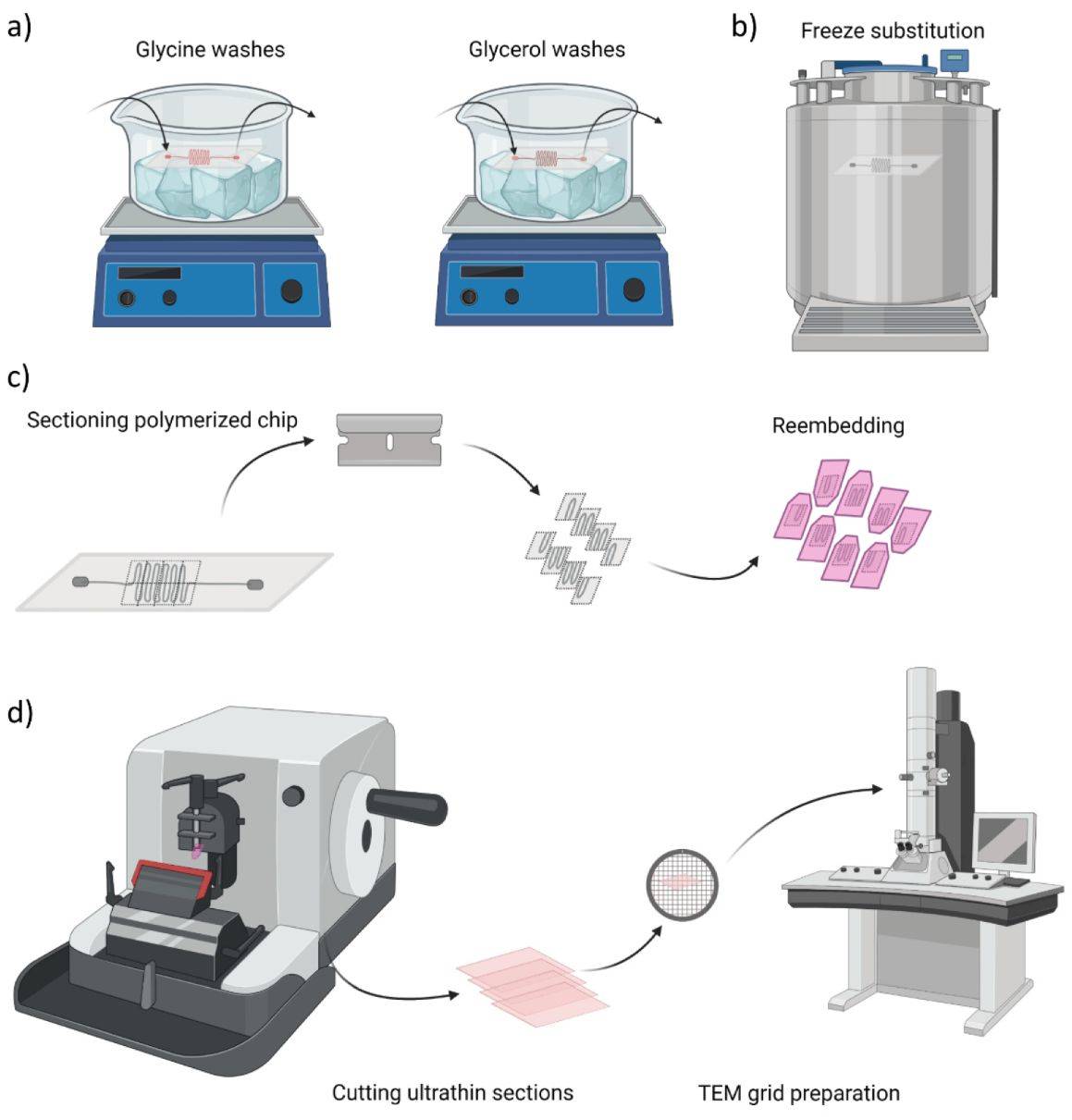 Figure 3. Overview of sample preparation for transmission electron microscopy (TEM) imaging. (Glinkowska Mares A, et al., 2021)
Figure 3. Overview of sample preparation for transmission electron microscopy (TEM) imaging. (Glinkowska Mares A, et al., 2021)
1. To obtain the thin slice specimen, the samples are first immobilized on a plastic material with glutaraldehyde or osmium tetroxide. These chemicals stabilize the structure of the cells and maintain their originality.
2. Add organic solvents (e.g., ethanol) to completely dehydrate the cells, and add unpolymerized liquid epoxy plastic to penetrate the sample and harden it.
3. Appropriate staining is applied to the sample to allow for uniform scattering of electrons. The sheet is then immersed in heavy metal elements, such as lead citrate and uranyl acetate, to allow the lean and aluminum ions to bind to the cell structures. An opaque layer of electrons is formed on the cell structure to increase contrast.
4. The stained thin section is then mounted on a copper grid for observation.
Extension of Transmission Electron Microscopy
| Technology | Description |
|
STEM is like the traditional TEM in that the image is formed by electrons passing through a sufficiently thin sample. Unlike TEM, in STEM, the electron beam is focused to a fine point and the sample is then scanned in a grating illumination system. It allows a direct correlation of image and spectral data. |
|
LVEM operates at relatively low electron acceleration voltages between 5-25 kV. The low voltage increases image contrast, significantly reduces or even eliminates the staining step, and allows for resolutions of several nanometers. |
|
Cryo-TEM uses a TEM with a sample holder that keeps the sample at liquid nitrogen or liquid helium temperature. This allows the sample to be prepared for imaging in glass ice, which is the preferred preparation technique for imaging individual molecules or macromolecular assemblies. |
|
Perform in-situ experiments in the TEM using a differential pumping chamber or specialized mounts to research nanomaterials, biological samples, molecular chemical reactions, and material deformation tests. |
|
Modern research TEMs may include an aberration corrector to reduce the amount of distortion in the image. Incident beam monochromators may also be used, which reduce the energy spread of the incident electron beam to less than 0.15eV. |
|
Using pulsed electrons it is possible to achieve temporal resolution well beyond the readout rate of the electron detector. Pulses can be generated by modifying the electron source for laser-triggered photoemission or by installing an ultrafast beam fader. |
Why Choose Transmission Electron Microscopy to Characterize Exosomes?
- Provides the highest, most powerful magnification of any microscope technology.
- Versatile imaging modes.
- Ability to collect electron diffraction patterns (crystal information) from nano-sized regions using Selected Area Diffraction (SAD).
- Capable of collecting localized information about composition and bonding that can be correlated with high-resolution images.
From exosome isolation, characterization, analysis, engineering, and application development, Creative Biostructure can customize solutions for client's specific research purposes.
If you are interested in our services and exosome products, please feel free to contact us. We look forward to working with you.
References
- Ahmed SS, et al. Nonparametric Denoising Methods Based on Contourlet Transform with Sharp Frequency Localization: Application to Low Exposure Time Electron Microscopy Images. Entropy. 2015. 17(5): 3461-3478.
- Franken LE, et al. A Technical Introduction to Transmission Electron Microscopy for Soft-Matter: Imaging, Possibilities, Choices, and Technical Developments. Small. 2020. 16(14): e1906198.
- Glinkowska Mares A, et al. Towards Cellular Ultrastructural Characterization in Organ-on-a-Chip by Transmission Electron Microscopy. Applied Nano. 2021. 2(4): 289-302.
- Chuo ST, et al. Imaging extracellular vesicles: current and emerging methods. J Biomed Sci. 2018. 25(1): 91.
- Pascucci L, Scattini G. Imaging extracellular vesicles by transmission electron microscopy: Coping with technical hurdles and morphological interpretation. Biochim Biophys Acta Gen Subj. 2021. 1865(4): 129648.