Structural Research of Phosphoglycosyl Transferases (PGT)
Phosphoglycosyl transferases (PGTs) are involved in a variety of glycosyl transfer reactions in cells, including the transfer of glycolic acid and phosphoglycans. Catalytic mechanisms, substrate specificity, and regulatory mechanisms can be revealed by solving their structures. In addition, phosphoglycosyl transferases play a significant role in the development and progression of many diseases, such as metabolic disorders and cancer. Understanding their structure is critical for the design and development of drugs targeting phosphoglycosyl transferases.
PGT catalyzes the transfer of the sugar-phosphate moiety from nucleoside diphosphate to poly (pentenyl phosphate) to form a membrane-bound pentenyl diphosphate product. PGT is an integral membrane protein with 1-11 predicted transmembrane structural domains. PGT has two structurally and mechanistically distinct superfamilies, the unitary PGT (monoPGT) and the multisite PGT (polyPGT), which catalyze the same transformation. Named for their topology relative to the membrane, monoPGT penetrates a single leaflet of the bilayer, whereas polyPGT contains multiple transmembrane helices. These enzyme superfamilies often coexist in a single organism, allowing the biosynthesis of different glycoconjugate products via different pathways.
The 2.7 Å crystal structure of a phosphoglucose transferase called PglC has been solved. The PGT-catalyzed reaction is a crucial step in assembling glycoconjugates, such as glycoproteins, glycolipids, and peptidoglycans, which are sugar-linked molecules required for bacterial survival and virulence. Studies have shown that PglC has three structural components. One part sticks out of the cell membrane, enters the aqueous cytoplasm of the cell and captures the hydrophilic molecule, the sugar nucleotide diphosphate. The second part is a helix with a curved serine-proline sequence that penetrates one half of the oily membrane bilayer where it binds the hydrophobic molecule poly(pentenyl phosphate). PglC then binds the two molecules together in the third part of the enzyme, which is located on the membrane surface and contains the active site.
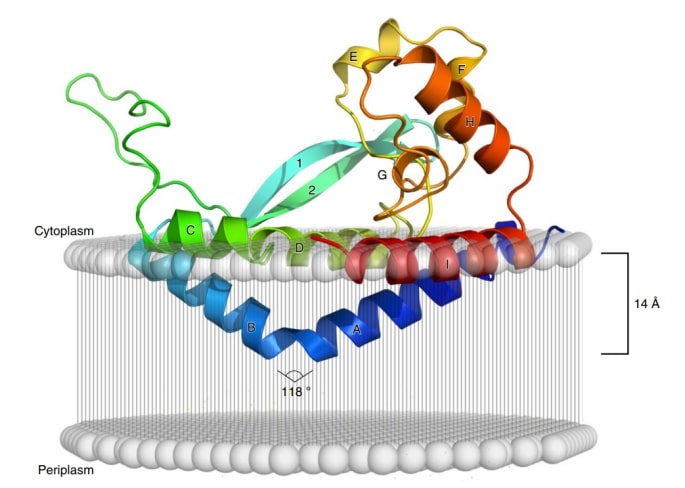 Figure 1. Model of PglC crystal structure sitting on a cell membrane. (Ray LC, et al., 2018).
Figure 1. Model of PglC crystal structure sitting on a cell membrane. (Ray LC, et al., 2018).
| Protein | Organism | Method | Resolution | PDB Entry ID |
| PglC phosphoglycosyl transferase, I57M / Q175M variant: Campylobacter concisus B (expressed in E. coli) | Campylobacter concisus 13826 | X-ray diffraction | 2.74 Å | 8G1N |
Table 1. Structural Research of Phosphoglycosyl Transferases (PGT).
As a specialist in structural biology, Creative Biostructure can provide services for structural studies of phosphoinositidase as well as many other types of protein. These services include crystallographic analysis, electron microscopy sample preparation and analysis, and NMR sample preparation and analysis. With our advanced equipment and professional team, we are committed to providing high-quality protein structural biology research services to our customers.
If you are interested in exploring structural studies of phosphoglucose transferase proteins and would like to learn more about the services we offer, please contact us. Our team is always available to discuss your research needs and provide the most effective solution for your project.
References
- O'Toole KH, et al. Glycoconjugate pathway connections revealed by sequence similarity network analysis of the monotopic phosphoglycosyl transferases. Proc Natl Acad Sci U S A. 2021; 118 (4).
- Ray LC, et al. Membrane association of monotopic phosphoglycosyl transferase underpins function. Nat Chem Biol. 2018; 14 (6): 538-541.
