Exosomes for ASO Delivery
Exosomes are small vesicles secreted by cells with diameters of about 30-150nm and a typical lipid bilayer structure, which play an essential role in intercellular material and information transfer. Due to low immunogenicity, high physicochemical stability, high tissue penetration, and an innate ability to transport, in recent years, scientists have begun to explore the application of exosomes for therapeutic molecule delivery, including antisense oligonucleotides (ASOs).
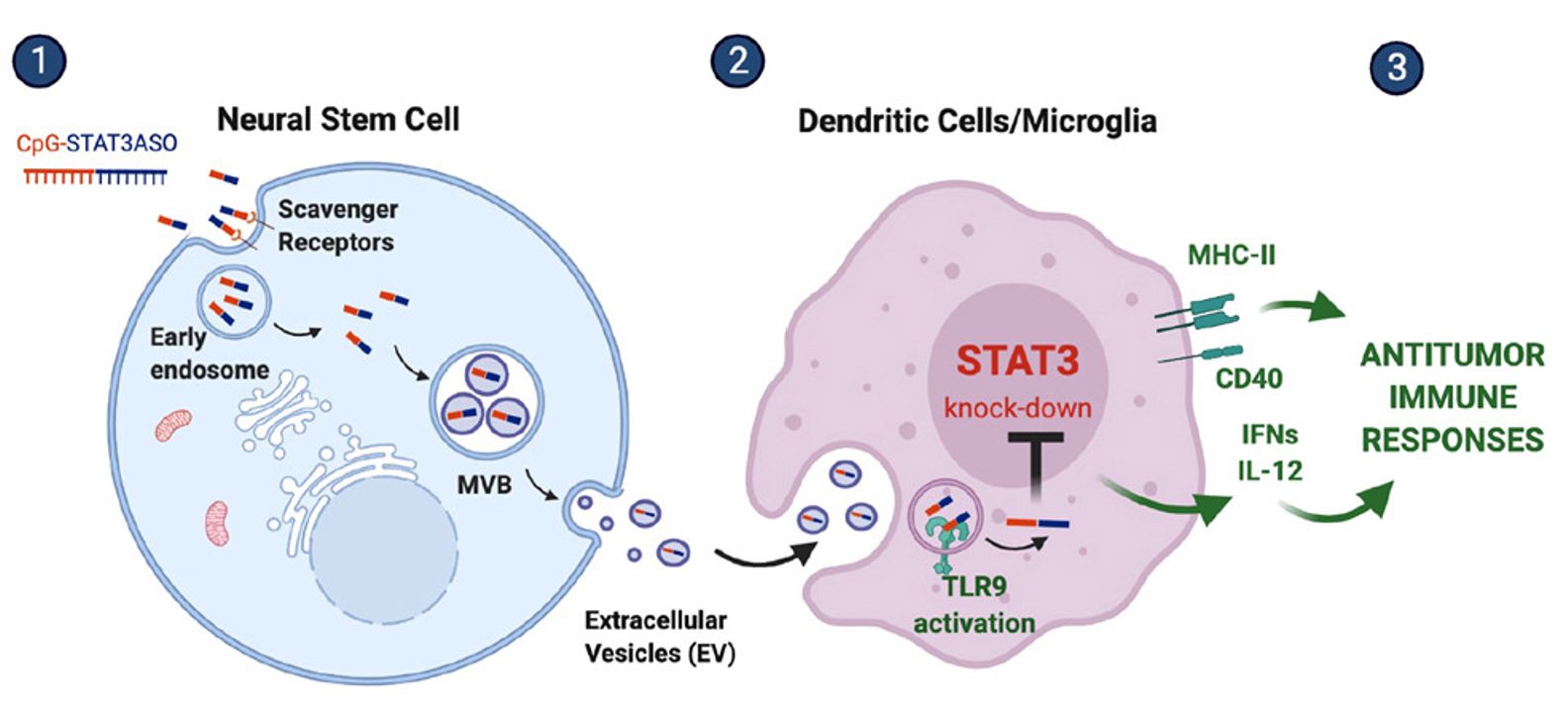 Figure 1. Schematic of exosome-encapsulated ASO delivery. (Adamus T, et al., 2021)
Figure 1. Schematic of exosome-encapsulated ASO delivery. (Adamus T, et al., 2021)
What is ASO?
ASO is a short-stranded nucleic acid (18-30nt) with a base order arrangement complementary to a specific target RNA sequence. Upon entering the cell, ASO binds to the RNA of the target gene and can regulate gene expression through a variety of mechanisms, including the formation of spatial site-blocking, the recruitment of RNA enzymes to degrade the mRNA, and the shearing off of exons. In recent years, ASO has developed into a novel platform for targeted cancer therapy. However, ASO molecules themselves are difficult to cross cell membranes and are easily degraded by proteases. Therefore, ASO needs to be loaded into cells via vectors, and exosomes are a novel and safer delivery method.
What is the Action Mechanism of ASO Drugs?
- RNase H-mediated degradation of RNA
RNase H1 (ribonuclease H1) is a ribonucleic acid endonuclease that specifically degrades RNA strands in RNA-DNA hybrids, thereby silencing the expression of target genes. The binding of DNA sequences to target sequences in ASO recruits RNase H-mediated degradation of RNA. Since RNase H is active in both the cytoplasm and the nucleus, ASO drugs based on this mechanism can target transcripts in the nucleus.
- Formation of spatial site-blocking to hinder protein translation
ASO drugs based on the spatial site-blocking mechanism can be combined with specific sequences in the transcripts, thus preventing or interfering with the mRNA, miRNA, or pre-mRNA from performing normal functions, both up-regulating/down-regulating gene expression.
a) The most widespread application of spatial site-blocking ASOs is to bind to pre-mRNAs and alter the splicing position of the spliceosome, thereby selectively excluding or retaining specific exons;
b) Spatial site-blocking ASOs can disrupt translation initiation by targeting the AUG start codon;
c) Some transcripts contain upstream open reading frames (uORFs) that modulate the translational activity of primary open reading frames (pORFs). Targeting uORF with spatial blocking ASO disrupts this regulation, leading to the activation of pORF translation.
d) Transcript stability can be regulated by altering the use of cleavage and polyadenylation signals.
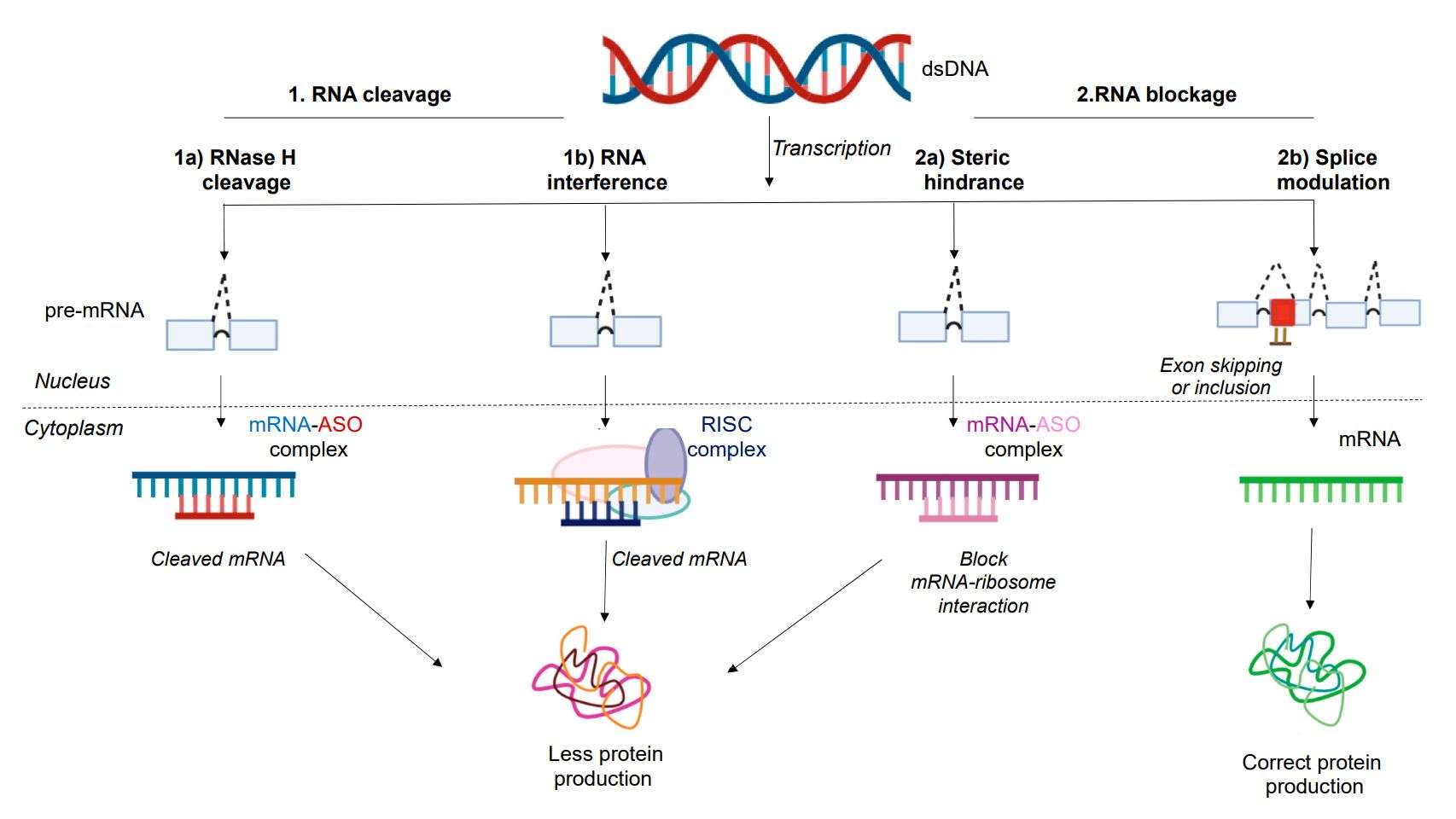 Figure 2. Mechanism of action of antisense oligonucleotides (ASOs). (Dhuri K, et al., 2020)
Figure 2. Mechanism of action of antisense oligonucleotides (ASOs). (Dhuri K, et al., 2020)
Exosome-based ASO Delivery
Achieving effective delivery of ASO therapeutics remains a major translational challenge. ASOs are typically large hydrophilic polyanions that do not readily cross the plasma membrane. To be active, systemically injected nucleic acid drugs must resist nuclease degradation in the extracellular space, bypass renal clearance, avoid plasma protein sequestration, avoid clearance by the reticuloendothelial system, escape the endolysosomal system, and reach the correct site of action. Systemic delivery to the central nervous system (CNS) presents additional hurdles because ASO-based therapies typically do not cross the blood-brain barrier.
Exosomes exhibit many favorable properties for ASO drug delivery:
- Exosomes can cross biological membranes.
- The presence of the marker protein CD47 protects exosomes from phagocytosis.
- Exosomes are considered non-toxic and have been used safely in graft-versus-host disease.
- Exosomes have the potential to be produced in an autologous manner.
- Some exosomes have been shown to have pro-regenerative and anti-inflammatory properties that can enhance therapeutic ASO delivery.
- Exosome engineering can be used as a modular platform for combining various therapies or targeted molecules.
Research Advances in Exosome-based ASO Delivery
Parkinson's disease (PD) is a common neurodegenerative disorder. Lewy bodies (LBs) form in the brains of patients, whose main component is phosphorylated and aggregated α-synuclein (α-syn). Research has found that ASO reduces α-syn expression. Using exosomes, scientists have developed a safe and efficient method of ASO delivery. Exosome-mediated delivery of ASO4 (exo-ASO4) showed high cellular uptake and low toxicity in primary neuronal cultures, and also significantly attenuated pre-formed α-syn aggregation in vitro. Injection of Exo-ASO4 into the brains of α-syn A53T mice, a transgenic model of PD, significantly reduced α-syn expression and attenuated its aggregation. In addition, the research found that exo-ASO4 ameliorated the degeneration of dopaminergic neurons in these mice, with a significant increase in motor function. Overall, this research suggests that exosome-mediated delivery of ASO4 may be an effective therapeutic option for PD.
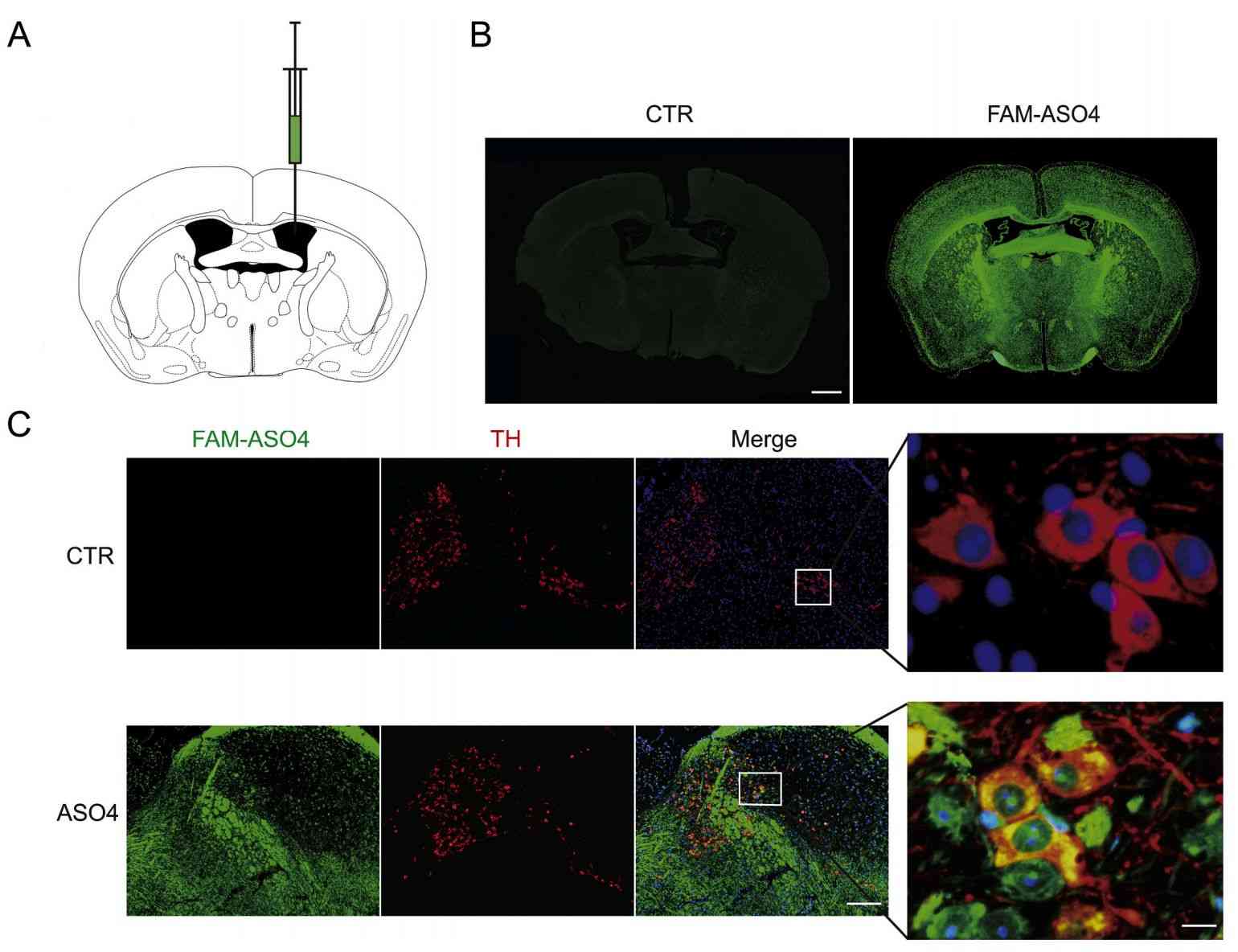 Figure 3. Distribution of exosome-delivered ASO4 in the mouse brain. (Yang J, et al., 2021)
Figure 3. Distribution of exosome-delivered ASO4 in the mouse brain. (Yang J, et al., 2021)
Tumor-associated macrophages (TAM) are potent drivers of the immunosuppressive tumor microenvironment and are attractive therapeutic targets. Researchers have developed novel exosomal therapeutic candidates that selectively deliver antisense oligonucleotides (ASO) to TAM, targeting critical transcription factors that control the immunosuppressive program. These transcription factors are activated by signaling molecules that lead to a tumor-promoting macrophage phenotype (M2). Exosomes loaded with STAT6 or C/EBPβ ASO (exoASO) induce dose-dependent knockdown (KD) of target genes in primary human M2 macrophages with greater potency than free ASO. Research has shown that exoASO therapy specifically targets and effectively reduces the expression of transcription factors in TAM, induces effective reprogramming, and produces potent antitumor activity.
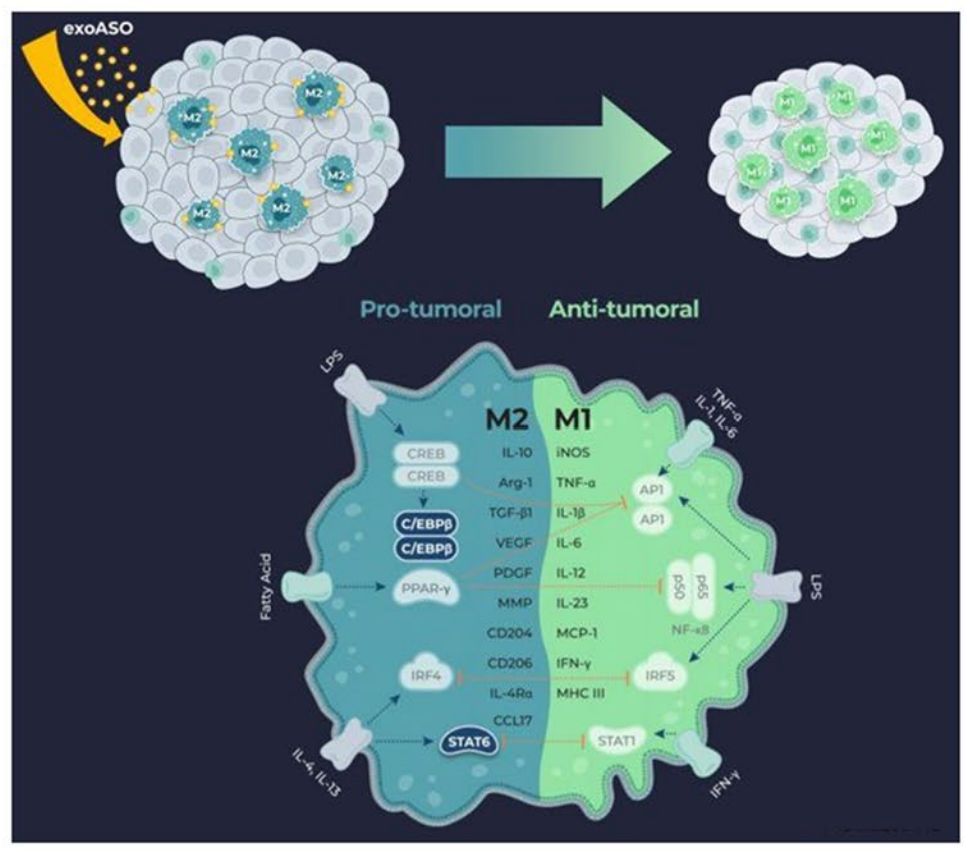 Figure 4. Engineered exosomes to deliver ASO to produce effective antitumor activity. (Zhang Y, et al., 2022)
Figure 4. Engineered exosomes to deliver ASO to produce effective antitumor activity. (Zhang Y, et al., 2022)
As a leading biological company, Creative Biostructure is committed to providing exosome cargo loading services. Our comprehensive services include exosome isolation, characterization, engineering, and functional analysis, combining cutting-edge technology with exceptional expertise to deliver reliable, superior results. If you are interested in our services, do not hesitate to contact us.
References
- Adamus T, et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol Ther Nucleic Acids. 2021. 27: 611-620.
- Dhuri K, et al. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J Clin Med. 2020. 9(6): 2004.
- Yang J, et al. Exosome-mediated delivery of antisense oligonucleotides targeting α-synuclein ameliorates the pathology in a mouse model of Parkinson's disease. Neurobiol Dis. 2021. 148: 105218.
- Zhang Y, et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J Nanobiotechnology. 2022. 20(1): 279.
- Roberts TC, et al. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020. 19(10): 673-694.
