Structural Research of Light-Harvesting Complexes
Light-harvesting complexes are crucial components in the photosynthetic process, enabling organisms to harness light energy for photochemical reactions. Among these fascinating complexes, diatoms stand out as a group of eukaryotic algae found in both fresh water and oceans, significantly contributing to ocean primary productivity by fixing substantial amounts of carbon dioxide into organic carbon. Their remarkable adaptability to the marine environment is attributed to their possession of specialized light-harvesting antennas called fucoxanthin (Fx) and chlorophyll (Chl) a/c-binding proteins (FCPs).
These pigment-protein assemblies play a pivotal role in capturing solar energy and funneling it to the photosynthetic reaction centers, where it is converted into chemical energy. Among various light-harvesting complexes, the investigation of FCPs in diatoms has been a subject of great interest. FCPs contain pigments Chl c and Fx, enabling them to absorb light in the blue-green region underwater, a part of the spectrum not effectively utilized by organisms containing only Chl a/b. The complex structure of FCPs offers unique insights into the efficient blue-green light harvesting and energy dissipation mechanisms in diatoms.
Recent advancements in structural biology techniques, such as X-ray crystallography and cryo-electron microscopy (cryo-EM), have revolutionized our ability to elucidate the intricate architecture of light-harvesting complexes. The determination of the FCP structure from diatoms has revealed a network of Chls a/c and Fxs, shedding light on the ligand structure and binding environment of each pigment. This crucial information will pave the way for in-depth studies on pigment absorption properties, energy transfer pathways, dynamics, and excess energy dissipation mechanisms within these antenna systems.
 Figure
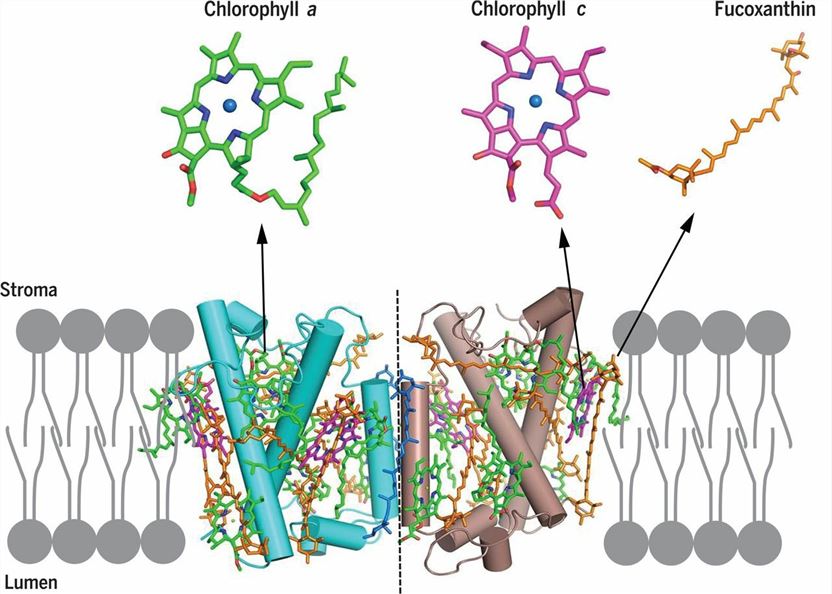
1. Structure of a FCP. (Wang W, et al., 2019)
Figure
1. Structure of a FCP. (Wang W, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Light-Harvesting Complex | Rhodoblastus acidophilus | X-ray diffraction | 2.50 Å | 1KZU |
| Light-Harvesting Complex | Rhodoblastus acidophilus | X-ray diffraction | 2.00 Å | 1NKZ |
| Light-Harvesting Complex | Magnetospirillum molischianum | X-ray diffraction | 2.40 Å | 1LGH |
| Light-Harvesting Complex LHC-II, Spinach Photosystem II | Spinacia oleracea | X-ray diffraction | 2.72 Å | 1RWT |
| Light-Harvesting Complex CP29, Spinach Photosystem II | Spinacia oleracea | X-ray diffraction | 2.80 Å | 3PL9 |
| Light-Harvesting Complex LHC-II, Pea Photosystem II | Pisum sativum | X-ray diffraction | 2.50 Å | 2BHW |
| Fucoxanthin and chlorophyll a/c complex | Phaeodactylum tricornutum | X-ray diffraction | 1.80 Å | 6A2W |
| Light-Harvesting Complex LHC-II | Marichromatium purpuratum | Cryo-EM single particle analysis | 2.38 Å | 6ZXA |
| Light-Harvesting Complex LHC-II | Cereibacter sphaeroides | Cryo-EM single particle analysis | 2.10 Å | 7PBW |
Table 1. Structural research of light-harvesting complexes.
As a leader in structural biology research, Creative Biostructure offers cutting-edge structural analysis services that have significantly contributed to advancing our understanding of light-harvesting complexes. Leveraging state-of-the-art technologies, including X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy (cryo-EM), our team of highly skilled scientists is committed to deciphering the molecular architecture of these intricate complexes.
Having accumulated years of experience in the industry, our team has effectively addressed numerous demanding projects, earning a reputation for excellence in structural biology research. Contact us to explore the ways our capabilities can empower your research endeavors and propel you closer to accomplishing your scientific aspirations.
References
- Wang W, et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science. 2019, 363(6427): eaav0365.
- Gardiner A T, et al. The 2.4 Å cryo-EM structure of a heptameric light-harvesting 2 complex reveals two carotenoid energy transfer pathways. Science Advances. 2021, 7(7): eabe4650.
- Qian P, et al. Cryo-EM structure of the Rhodobacter sphaeroides light-harvesting 2 complex at 2.1 Å. Biochemistry. 2021, 60(44): 3302-3314.