Structural Research of Forespore Sporulation Channels
Bacteria use specialized protein secretion systems to export proteins to the extracellular environment. Some identified protein secretion systems are composed of large complexes, which gather in and around the bacterial membrane to form special channels. Through these channels, selected proteins are actively delivered. Forespore sporulation channels are a class of important proteins that participate in the key channels in the formation of Bacterial spore.
The formation of Bacterial spores allows hungry cells to differentiate into metabolic dormant spores, which can survive under extreme conditions. After asymmetric division, the mother cell engulfs the prespore and surrounds it with two bilayer membranes. During the engulfment process, an important channel, known as the feed tube device, is considered to pass through two membranes and form a direct channel between the mother cell and the prespore. Creating this channel requires at least nine proteins, including SpoIIQ and SpoIIIAA-AH.
The near-atomic resolution structure of SpoIIIAG is determined by single-particle low-temperature EM. The near-atomic resolution structure clearly indicates that SpoIIIAG exhibits adaptability to RBM folding and has a unique β Triangle insertion, which is assembled into a protruding channel. Its size indicates the pre-spores of macromolecules in the potential channel feeding process between the mother cell and the cell. 3D reconstruction shows that SpoIIIAG is assembled into a large and stable 30-fold symmetric complex with a unique mushroom-like structure. The composite consists of three unique circular structures: a 60-strand vertical structure β Bucket, forming a large internal channel surrounded by two concentric rings, one composed of β Mediated by another repetitive ring construction motif (RBM), a common structure of various bimembrane secretion systems with different functions is formed.
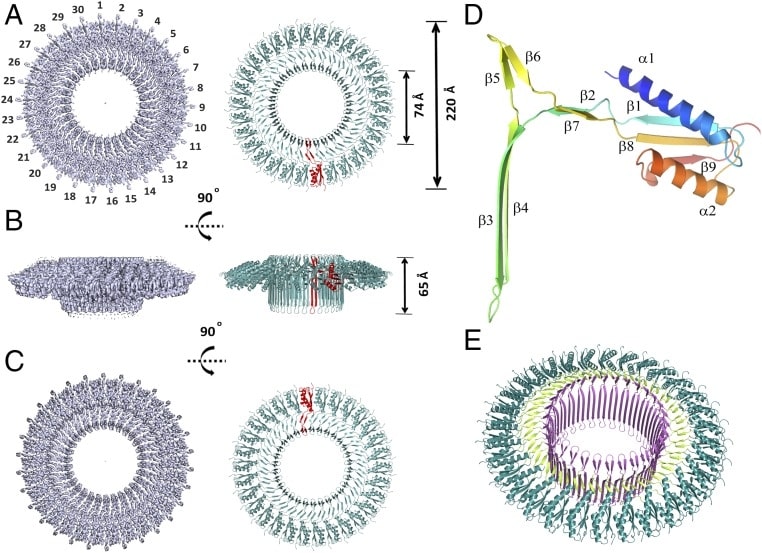 Figure 1. Cryo-EM structure of SpoIIIAG55-end. (Zeytuni N, et al., 2017)
Figure 1. Cryo-EM structure of SpoIIIAG55-end. (Zeytuni N, et al., 2017)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| SpoIIIAG sporolation channel: Bacillus subtilis (expressed in E. coli) | Bacillus subtilis BEST7613 | Cryo-EM single particle analysis | 3.5 Å | 5WC3 |
Table 1. Structural Research of Forespore Sporulation Channels.
The structural analysis of forespore sporulation channels is of great significance for revealing the regulatory mechanism during the formation of bacterial spore. The results of structural research help to understand the functional mechanism, signal transduction pathways, and interactions with other proteins of the forespore sporulation channels in spore formation. At the same time, structural research can also provide potential targets and strategies for developing new antibacterial drugs and controlling bacterial infections.
Creative Biostructure is a leading biotechnology company focusing on the protein structural biology. We provide foreshore sporulation channels and various types of protein structure research related services, such as X-ray crystallography, cryo-electron microscopy (Cryo-EM), and nuclear magnetic resonance (NMR) services. Our professional team has rich experience and advanced equipment, which can provide comprehensive support for your research. Our team is committed to promoting the development of science and can provide innovative solutions to solving problems such as antibacterial drug development and infection control. If you are interested in protein structure analysis and related research fields, please feel free to contact us for more detailed information.
Reference
- Zeytuni N, et al. Near-atomic resolution cryoelectron microscopy structure of the 30-fold homooligomeric SpoIIIAG channel essential to spore formation in Bacillus subtilis. Proc Natl Acad Sci U S A. 2017. 114 (34): E7073-E7081.