Protein Stability Testing
Proteins, the workhorses of biological systems, play pivotal roles in cellular structure, enzymatic reactions, and signaling pathways. In biopharmaceuticals, where proteins are harnessed as therapeutic agents, their stability is a critical determinant of safety, efficacy, and manufacturability. Protein stability testing is the systematic evaluation of a protein's resilience under various physical, chemical, and environmental stresses.
At Creative Biostructure, we specialize in providing comprehensive stability assay services tailored to your needs. Our offerings include advanced techniques such as Differential Scanning Calorimetry (DSC), Protein Thermal Shift Assay (PTSA), and Fourier Transform Infrared Spectroscopy (FTIR).
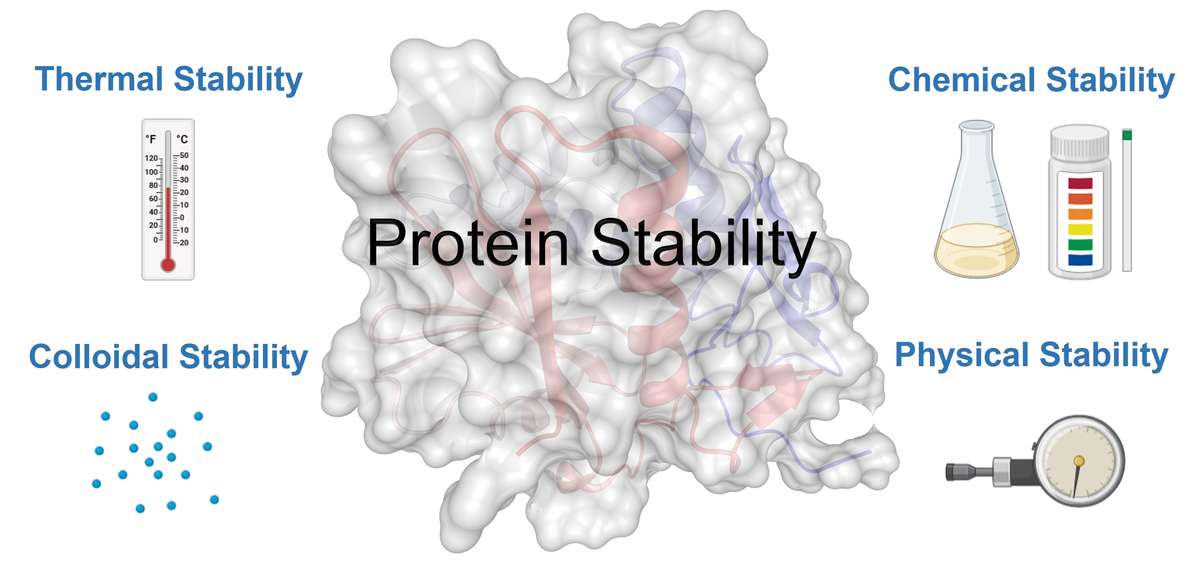
Understanding Protein Stability
Protein stability refers to the ability of a protein to maintain its structural integrity and functional activity under physiological and non-physiological conditions. It encompasses several dimensions, including:
- Thermal Stability: The protein's resistance to denaturation at elevated temperatures. This aspect is crucial for proteins exposed to temperature variations during manufacturing, storage, or therapeutic use.
- Chemical Stability: The ability to withstand changes in pH, ionic strength, or the presence of denaturants. This involves resistance to chemical degradation pathways such as oxidation, deamidation, or hydrolysis, which can compromise functionality and safety.
- Colloidal Stability: The propensity of a protein to remain soluble without forming aggregates. Aggregation not only reduces therapeutic efficacy but also poses immunogenic risks, making colloidal stability a cornerstone for biopharmaceutical formulations.
- Physical Stability: The structural integrity of a protein against mechanical forces such as agitation, shear stress, or freeze-thaw cycles. Understanding physical stability is vital for developing robust handling and storage protocols.
Each of these dimensions is interdependent, contributing collectively to the overall stability profile of a protein. Comprehensive understanding and testing of these aspects are essential to ensure that proteins function as intended during production, storage, and administration, safeguarding their therapeutic or diagnostic utility.
Thermal Stability Testing
Thermal stability is a critical parameter in protein stability testing, especially for biopharmaceutical formulations. It assesses the ability of a protein to withstand elevated temperatures without undergoing irreversible denaturation.
Techniques for Thermal Stability Testing
- Differential Scanning Calorimetry (DSC): DSC measures the heat flow associated with protein unfolding. The melting temperature (Tm) is a key output, representing the temperature at which 50% of the protein population is denatured. Higher Tm values typically indicate greater thermal stability.
- Protein Thermal Shift Assays (PTSA): PTSA monitors the unfolding of proteins using a fluorescent dye that binds to hydrophobic regions exposed during denaturation. This technique is high-throughput and particularly useful for screening stabilizers.
- Dynamic Light Scattering (DLS): DLS detects changes in particle size distribution as proteins aggregate at elevated temperatures. It provides insights into both thermal stability and colloidal stability.
- Circular Dichroism (CD) Spectroscopy: CD spectroscopy measures changes in protein secondary structure upon heating. It is particularly useful for characterizing α-helix and β-sheet content.
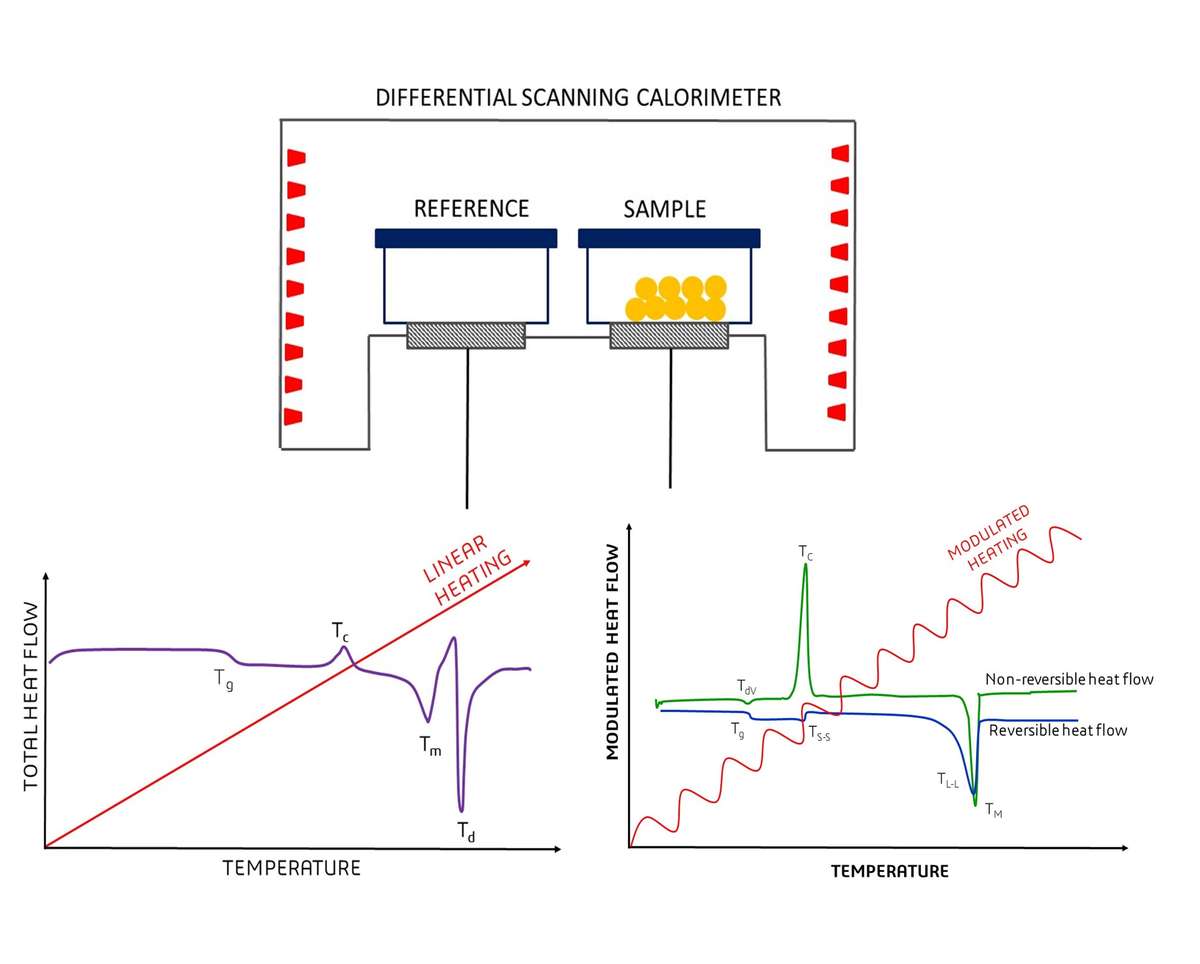 Figure 1. Overview of Differential Scanning Calorimetry. (Adapted from Leyva-Porras et al., 2019)
Figure 1. Overview of Differential Scanning Calorimetry. (Adapted from Leyva-Porras et al., 2019)
Chemical Stability Testing
Proteins are susceptible to chemical modifications that can affect their stability and function. Chemical stability testing involves exposing proteins to various chemical stressors and monitoring their effects.
Key Chemical Stressors
- pH Variations: Proteins have an optimal pH range where their structure and activity are preserved. Deviations from this range can lead to denaturation or aggregation.
- Oxidation: Oxidative stress, often caused by reactive oxygen species, can modify amino acid residues, particularly methionine and cysteine.
- Deamidation: The conversion of asparagine residues to aspartic acid under physiological conditions is a common degradation pathway.
- Hydrolysis: Acidic or basic conditions can cleave peptide bonds, leading to fragmentation.
Analytical Methods for Chemical Stability
- High-Performance Liquid Chromatography (HPLC): Detects and quantifies chemical modifications and fragments.
- Mass Spectrometry (MS): Provides detailed insights into structural changes at the molecular level.
- UV-Vis Spectroscopy: Monitors changes in aromatic amino acid residues indicative of chemical degradation.
 Figure 2. High-Performance Liquid Chromatography (HPLC) equipment.
Figure 2. High-Performance Liquid Chromatography (HPLC) equipment.
Colloidal Stability Testing
Colloidal stability reflects the tendency of a protein to remain in solution without forming aggregates. Aggregation is a major concern in biopharmaceuticals due to its potential to reduce efficacy and increase immunogenicity.
Techniques for Colloidal Stability Testing
- Dynamic Light Scattering (DLS): Measures the hydrodynamic radius of proteins and detects the formation of aggregates in solution.
- Size-Exclusion Chromatography (SEC): Separates aggregated species from monomeric proteins, providing quantitative data on aggregation levels.
- Analytical Ultracentrifugation (AUC): Distinguishes between monomers, oligomers, and aggregates based on sedimentation velocity.
- Visual Inspection: While less quantitative, visual inspection under light microscopy can detect large aggregates or particulates.
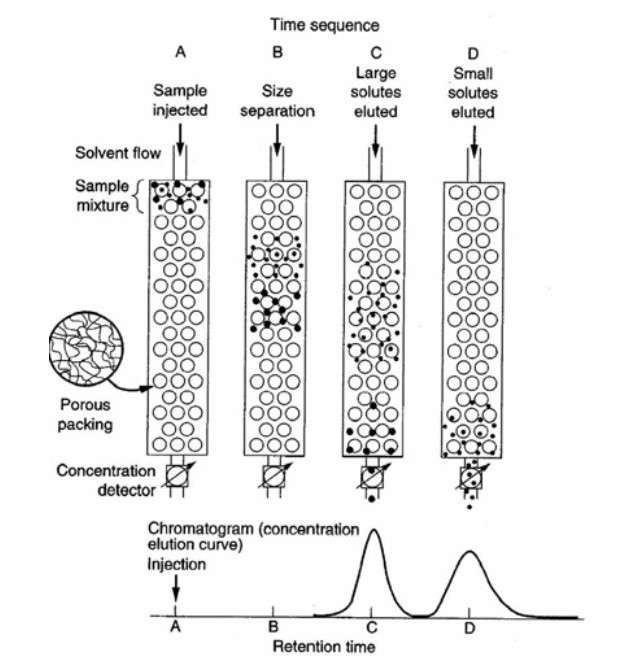 Figure 3. Mechanism of size-exclusion chromatography (SEC). (Yau et al., 1979)
Figure 3. Mechanism of size-exclusion chromatography (SEC). (Yau et al., 1979)
Physical Stability Testing
Physical stability testing examines the impact of external mechanical forces on protein integrity. Proteins are often subjected to agitation, shear stress, or freeze-thaw cycles during production and storage.
Common Physical Stressors
- Agitation: Shaking or stirring can induce aggregation, particularly for surface-active proteins.
- Freeze-Thaw Cycles: Rapid temperature changes can cause ice crystal formation, leading to protein denaturation or aggregation.
- Mechanical Shear: Shear forces in pumps or syringes can disrupt protein structures.
Testing Methods
- Freeze-Thaw Stability Assays: Involves repeated freezing and thawing cycles, with subsequent analysis by DLS or SEC.
- Agitation Studies: Monitors aggregation induced by vortexing or stirring.
- Microflow Imaging (MFI): Visualizes subvisible particles formed under physical stress.
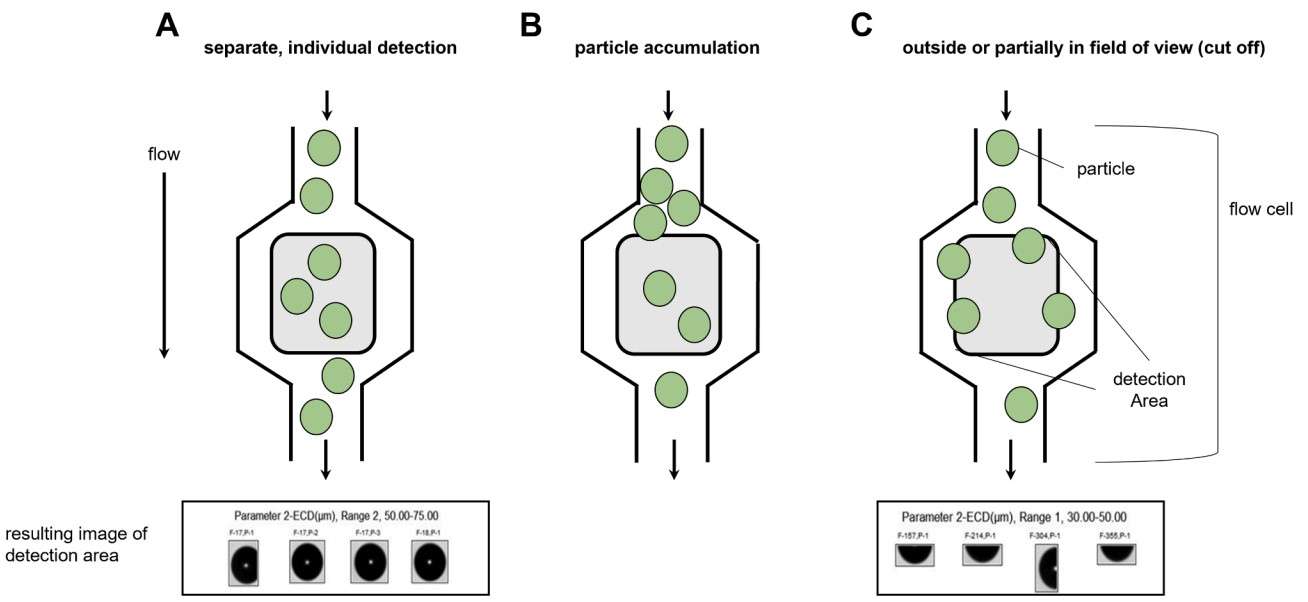 Figure 4. Schematic drawing of the MFI™ flow cell as indicated. The detection area of 80–85 % is shaded in grey. Particles passing the cell are depicted as green spheres for an ideal scenario where particles passing separately through the flow cell (A), and thus are fully detected individually. (B) Accumulation of particles at the port of the MFI™ flow cell. Particle flow is impaired due to accumulation. Blockage of the flow cell can result in lower particle level. (C) Cut-off scenario. The MFI™ flow cell is not fully covered by detection resulting in only 80–85 % coverage. Consequently, larger particles can be incompletely or only partially detected as they are cut-offed within the detection area. (Fawaz et al., 2023)
Figure 4. Schematic drawing of the MFI™ flow cell as indicated. The detection area of 80–85 % is shaded in grey. Particles passing the cell are depicted as green spheres for an ideal scenario where particles passing separately through the flow cell (A), and thus are fully detected individually. (B) Accumulation of particles at the port of the MFI™ flow cell. Particle flow is impaired due to accumulation. Blockage of the flow cell can result in lower particle level. (C) Cut-off scenario. The MFI™ flow cell is not fully covered by detection resulting in only 80–85 % coverage. Consequently, larger particles can be incompletely or only partially detected as they are cut-offed within the detection area. (Fawaz et al., 2023)
Emerging Techniques in Protein Stability Testing
Advancements in analytical technologies are revolutionizing protein stability testing. Emerging techniques include:
- Native Mass Spectrometry: Allows detailed analysis of protein stability under near-native conditions, preserving structural and functional integrity during evaluation. This technique can detect subtle conformational changes and provides insight into the effects of modifications and interactions on protein stability.
- Fourier-Transform Infrared (FTIR) Spectroscopy: Provides comprehensive insights into secondary structure alterations by detecting changes in protein absorption bands. This is crucial for understanding unfolding, aggregation, or other structural transformations during formulation or stress conditions.
- Microcalorimetry: Delivers ultra-sensitive measurements of thermal transitions, enabling precise determination of heat capacity changes and denaturation temperatures. This is particularly useful for assessing the thermodynamic stability of biopharmaceutical proteins.
- High-Throughput Screening (HTS): Automates the stability evaluation process, using miniaturized setups and robotics to accelerate the evaluation of multiple formulations or conditions. HTS accelerates discovery and optimization processes by providing fast and robust data generation.
These techniques represent a significant leap forward in sensitivity, specificity, and scalability, addressing the increasing demands of complex protein therapeutics and paving the way for innovative solutions in biopharmaceutical development.
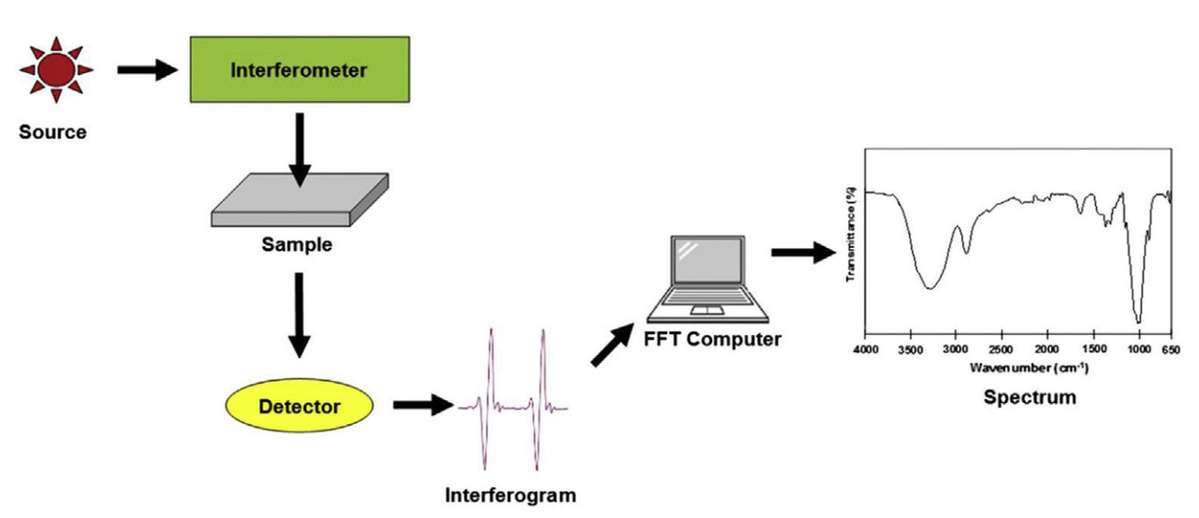 Figure 5. FTIR processing. (Undavalli et al., 2021)
Figure 5. FTIR processing. (Undavalli et al., 2021)
Applications of Protein Stability Testing
Drug Development
Stability testing ensures that therapeutic proteins retain their biological activity and safety throughout their lifecycle. This includes rigorous analysis during formulation, storage, and transportation to meet regulatory standards.
Bioprocess Optimization
By identifying the optimal conditions for protein production and purification, stability testing enhances yield, reduces costs, and ensures product consistency in large-scale manufacturing processes.
Vaccine Formulation
Stability testing ensures that antigens retain their structural integrity and immunogenic properties, which are critical for an effective immune response. This is particularly important for vaccines distributed in regions with limited cold chain infrastructure.
Diagnostic Reagents
Protein stability testing ensures the reliability and shelf-life of protein-based diagnostic kits. Stable diagnostic reagents are crucial for accurate disease detection and monitoring.
Agricultural and Industrial Applications
In non-medical areas, stability testing facilitates the development of enzymes and other protein-based solutions for agriculture and industry, ensuring their functionality under a variety of environmental conditions.
As a leading provider of assay services, Creative Biostructure offers a wide range of protein stability testing solutions, including Differential Scanning Calorimetry (DSC), Protein Thermal Shift Assay (PTSA), and Size-Exclusion Chromatography. Contact us today to discover how our expertise can support your project needs.
References
- Bischof JC, He X. Thermal stability of proteins. Annals of the New York Academy of Sciences. 2006;1066(1):12-33.
- Fawaz I, Schaz S, Boehrer A, Garidel P, Blech M. Micro-flow imaging multi-instrument evaluation for sub-visible particle detection. European Journal of Pharmaceutics and Biopharmaceutics.2023;185:55-70.
- Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solís V, et al. Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers. 2019;12(1):5.
- Panowicz R, Niezgoda T, Palka N, Niezgoda T. The initial results of THz spectroscopy non-destructive investigations of epoxy-glass composite structure. Computer Methods in Mechanics. 2011.
- Timr S, Madern D, Sterpone F. Protein thermal stability. In: Progress in Molecular Biology and Translational Science. Vol 170. Elsevier; 2020:239-272.
- Undavalli VK, Ling C, Khandelwal B. Impact of alternative fuels and properties on elastomer compatibility. In: Aviation Fuels. Elsevier; 2021:113-132.
- Yau WW, Kirkland JJ, Bly DD, Yau WW, Kirkland JJ, Bly DD. Modern Size-Exclusion LiquidChromatography: Practice of Gel Permeation and Gel Filtration Chromatography. Wiley; 1979.