Structural Research of Electron Transport Chain Cytochrome b6f Complex
The cytochrome b6f (cytb6f) complex plays a vital role in the process of photosynthesis. It functions as a link between photosystems I and II, converting solar energy into a proton gradient that is used for ATP synthesis. Additionally, the cytb6f complex is also a redox-sensing hub in higher plants, regulating light harvesting and cyclic electron transfer to protect against environmental stress.
The advancement of structural biology techniques has greatly contributed to our understanding of the molecular basis of biological processes. Cryo-electron microscopy (cryo-EM) is one such technique that has revolutionized the field by enabling the visualization of large and complex macromolecular structures at near-atomic resolutions.
To understand the structural basis for the operation of the quinol (Q) cycle and the redox-sensing function of cytb6f, a cryo-EM structure of the dimeric cytb6f complex from spinach was determined at a resolution of 3.6 Å. According to the revealed structure, the cytb6f complex contains up to three plastoquinone (PQ) molecules. PQ1 was found near the PQ oxidation site (Qp) in one of the cytb6f monomers and was located adjacent to haem bp and chlorophyll a. Interestingly, two different conformations of the chlorophyll a phytyl tail were discovered, suggesting a gating function for chlorophyll a in redox sensing. PQ2 was observed to partially obstruct the PQ reduction site (Qn) on the PQ1 side, committing the electron transfer network to turnover at the occupied Qn site in the neighboring monomer. Furthermore, a conformational switch involving the haem cn propionate was found to promote two-electron, two-proton reduction at the Qn site, thereby avoiding the formation of the reactive intermediate semiquinone. Finally, a third PQ molecule was tentatively located, which is consistent with a transition between the Qp and Qn sites in opposite monomers during the Q cycle. By revealing the detailed structure of the complex, these studies have shed light on the mechanism of electron transfer within cytb6f, particularly via the Q cycle.
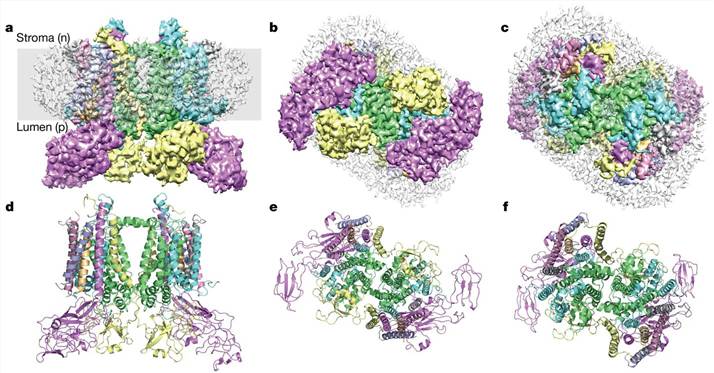 Figure 1. Cryo-EM structure of the cytb6f complex from spinach. (Malone L A, et al., 2019)
Figure 1. Cryo-EM structure of the cytb6f complex from spinach. (Malone L A, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Cytochrome b6f complex | Mastigocladus laminosus | X-ray diffraction | 3.00 Å | 1VF5 |
| Cytochrome b6f complex | Mastigocladus laminosus | X-ray diffraction | 3.80 Å | 2D2C |
| Cytochrome b6f complex, native structure | Mastigocladus laminosus | X-ray diffraction | 3.00 Å | 2E74 |
| Cytochrome b6f complex with bound TDS | Mastigocladus laminosus | X-ray diffraction | 3.07 Å | 4H13 |
| Cytochrome b6f complex (expressed in Chlamydomonas reinhardtii) | Chlamydomonas reinhardtii | X-ray diffraction | 3.10 Å | 1Q90 |
| Cytochrome b6f complex | Nostoc sp. PCC 7120 | X-ray diffraction | 3.00 Å | 2ZT9 |
| Cytochrome b6f complex | Nostoc sp. PCC 7120 | X-ray diffraction | 2.70 Å | 4H44 |
| Dimeric cytochrome b6f complex with bound thylakoid lipids and plastoquinones | Spinacia oleracea | Cryo-EM single particle analysis | 3.58 Å | 6RQF |
| Dimeric cytochrome b6f complex with bound plastoquinones | Spinacia oleracea | Cryo-EM single particle analysis | 2.70 Å | 7QRM |
Table 1. Structural Research of Cytochrome b6f of Oxygenic Photosynthesis.
At Creative Biostructure, we provide advanced cryo-electron microscopy (cryo-EM) and X-ray crystallography services to support structural biology research. Our experienced team of scientists has extensive expertise in solving complex macromolecular structures at high resolution, including membrane proteins such as cytb6f. Our cryo-EM equipment is equipped with the latest sample preparation, data acquisition, and image processing instruments. Our X-ray crystallography services include protein crystallization, crystal optimization, data collection, structure determination, and refinement. Our advanced structural analysis services allow us to provide detailed information about the characteristics of our clients' target macromolecular structures, including conformational changes, ligand binding, and intermolecular interactions.
Our strength lies in our commitment to quality, speed, and cost-effectiveness. We combine cutting-edge technology, advanced software, and state-of-the-art instruments to provide high-quality structural data in a timely and economical manner. Our team works closely with clients, providing detailed reports and consultation throughout the entire project process to help them understand the structural insights gained from our analysis. Contact us now to learn more about our structural analysis services and how we can help you achieve your research goals.
References
- Malone L A, et al. Cryo-EM structure of the spinach cytochrome b 6 f complex at 3.6 Å resolution. Nature. 2019, 575(7783): 535-539.
- Sarewicz M, et al. High-resolution cryo-EM structures of plant cytochrome b6f at work. Science Advances. 2023, 9(2): eadd9688.